Polarity of varicosity initiation in central neuron mechanosensation
- PMID: 28606925
- PMCID: PMC5496611
- DOI: 10.1083/jcb.201606065
Polarity of varicosity initiation in central neuron mechanosensation
Abstract
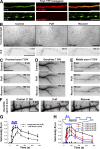
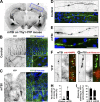
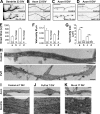
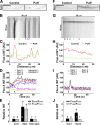
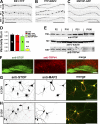
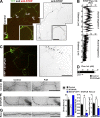
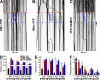
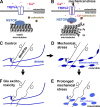
Little is known about mechanical regulation of morphological and functional polarity of central neurons. In this study, we report that mechanical stress specifically induces varicosities in the axons but not the dendrites of central neurons by activating TRPV4, a Ca2+/Na+-permeable mechanosensitive channel. This process is unexpectedly rapid and reversible, consistent with the formation of axonal varicosities in vivo induced by mechanical impact in a mouse model of mild traumatic brain injury. In contrast, prolonged stimulation of glutamate receptors induces varicosities in dendrites but not in axons. We further show that axonal varicosities are induced by persistent Ca2+ increase, disassembled microtubules (MTs), and subsequently reversible disruption of axonal transport, and are regulated by stable tubulin-only polypeptide, an MT-associated protein. Finally, axonal varicosity initiation can trigger action potentials to antidromically propagate to the soma in retrograde signaling. Therefore, our study demonstrates a new feature of neuronal polarity: axons and dendrites preferentially respond to physical and chemical stresses, respectively.
© 2017 Gu et al.
Figures


Similar articles
-
Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury.Exp Neurol. 2012 Jan;233(1):364-72. doi: 10.1016/j.expneurol.2011.10.030. Epub 2011 Nov 4. Exp Neurol. 2012. PMID: 22079153 Free PMC article.
-
Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation.J Neurosci. 2016 Jan 27;36(4):1071-85. doi: 10.1523/JNEUROSCI.2430-15.2016. J Neurosci. 2016. PMID: 26818498 Free PMC article.
-
Dendrites differ from axons in patterns of microtubule stability and polymerization during development.Neural Dev. 2009 Jul 14;4:26. doi: 10.1186/1749-8104-4-26. Neural Dev. 2009. PMID: 19602271 Free PMC article.
-
Rapid and Reversible Development of Axonal Varicosities: A New Form of Neural Plasticity.Front Mol Neurosci. 2021 Feb 3;14:610857. doi: 10.3389/fnmol.2021.610857. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33613192 Free PMC article. Review.
-
Mini-review: Microtubule sliding in neurons.Neurosci Lett. 2021 May 14;753:135867. doi: 10.1016/j.neulet.2021.135867. Epub 2021 Apr 1. Neurosci Lett. 2021. PMID: 33812935 Free PMC article. Review.
Cited by
-
Outcomes of the 2019 hydrocephalus association workshop, "Driving common pathways: extending insights from posthemorrhagic hydrocephalus".Fluids Barriers CNS. 2023 Jan 13;20(1):4. doi: 10.1186/s12987-023-00406-7. Fluids Barriers CNS. 2023. PMID: 36639792 Free PMC article. Review.
-
Mitochondrial behavior when things go wrong in the axon.Front Cell Neurosci. 2022 Aug 5;16:959598. doi: 10.3389/fncel.2022.959598. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35990893 Free PMC article. Review.
-
Actomyosin-II protects axons from degeneration induced by mild mechanical stress.J Cell Biol. 2024 Aug 5;223(8):e202206046. doi: 10.1083/jcb.202206046. Epub 2024 May 7. J Cell Biol. 2024. PMID: 38713825 Free PMC article.
-
Acute axon damage and demyelination are mitigated by 4-aminopyridine (4-AP) therapy after experimental traumatic brain injury.Acta Neuropathol Commun. 2022 May 2;10(1):67. doi: 10.1186/s40478-022-01366-z. Acta Neuropathol Commun. 2022. PMID: 35501931 Free PMC article.
-
Experimental Traumatic Brain Injury Identifies Distinct Early and Late Phase Axonal Conduction Deficits of White Matter Pathophysiology, and Reveals Intervening Recovery.J Neurosci. 2018 Oct 10;38(41):8723-8736. doi: 10.1523/JNEUROSCI.0819-18.2018. Epub 2018 Aug 24. J Neurosci. 2018. PMID: 30143572 Free PMC article.
References
-
- Andrieux A., Salin P.A., Vernet M., Kujala P., Baratier J., Gory-Fauré S., Bosc C., Pointu H., Proietto D., Schweitzer A., et al. . 2002. The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders. Genes Dev. 16:2350–2364. 10.1101/gad.223302 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

