RPA activates the XPF-ERCC1 endonuclease to initiate processing of DNA interstrand crosslinks
- PMID: 28607004
- PMCID: PMC5510000
- DOI: 10.15252/embj.201796664
RPA activates the XPF-ERCC1 endonuclease to initiate processing of DNA interstrand crosslinks
Abstract
During replication-coupled DNA interstrand crosslink (ICL) repair, the XPF-ERCC1 endonuclease is required for the incisions that release, or "unhook", ICLs, but the mechanism of ICL unhooking remains largely unknown. Incisions are triggered when the nascent leading strand of a replication fork strikes the ICL Here, we report that while purified XPF-ERCC1 incises simple ICL-containing model replication fork structures, the presence of a nascent leading strand, modelling the effects of replication arrest, inhibits this activity. Strikingly, the addition of the single-stranded DNA (ssDNA)-binding replication protein A (RPA) selectively restores XPF-ERCC1 endonuclease activity on this structure. The 5'-3' exonuclease SNM1A can load from the XPF-ERCC1-RPA-induced incisions and digest past the crosslink to quantitatively complete the unhooking reaction. We postulate that these collaborative activities of XPF-ERCC1, RPA and SNM1A might explain how ICL unhooking is achieved in vivo.
Keywords: RPA; Fanconi anaemia; SNM1A; XPF‐ERCC1; interstrand crosslinks.
© 2017 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

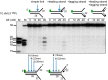
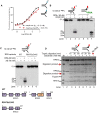
Purified XPF‐ERCC1 (XE) analysed on an SDS–PAGE gel (4–12%) stained with InstantBlue (left‐hand panel) and Western blot analysis (right‐hand panel). WT = wild type XE; D676A = XE mutated to substitute aspartic acid residue 676 of XPF with alanine.
Nuclease activity of WT and D676A forms of XE on a “simple fork” substrate. The substitution of metal‐binding residue in XPF (D676A) renders the XE complex devoid of any nuclease activity. Red circles denote 3′[32P]‐radiolabelled nucleotides.
(Top panel) Nuclease activity of XE on a “simple fork” substrate over a time course. (Bottom panel) Quantification of intact substrate and incision products expressed as a percentage of initial substrate as in top panel, n = 2.

Nuclease activity of XE on “fork‐like” DNA substrates (simple fork; +leading strand; +lagging strand; +leading and +lagging strands). The red circles denote 3′[32P]‐radiolabelled nucleotides. “M” denotes molecular weight marker.
A schematic representation of XE incisions on a “simple fork” and “+leading‐strand” substrates. The positions of incision with respect to the fork junctions are indicated in bold (2 or 6 nt from the fork junction), and size of 3′[32P]‐labelled incision products and percentage of incisions are indicated in parentheses.
Quantification of total substrate incisions expressed as a percentage of initial substrate as in (B). Unpaired two‐tailed t‐test; **P < 0.01. Error bars represent SEM, n = 3.
Nuclease activity of XE on fork substrates with increasing length of a model nascent leading strand (simple fork; −9; −3; −2; −1; 0 nt from the fork junction).
A schematic representation of XPF‐ERCC1 incisions as in (D).
Quantification of intact substrate and incision products expressed as a percentage of initial substrate at 40 nM XE as in (D). Error bars represent SEM, n = 3.
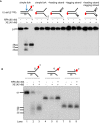
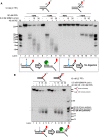
The nuclease activity of XE in the presence and absence of RPA on fork substrates 3′[32P]‐radiolabelled either on the “top” or the “bottom” strand. XPF‐ERCC1 activity is not detectable on the bottom strand of the fork substrates, which have a 5′‐ssDNA arm.
The nuclease activity of XE in the presence and absence of RPA on simple fork substrates containing arms of 23, 13 or 3 nucleotides.
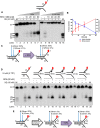
Nuclease activity of XE on a “simple fork” with increasing concentration of RPA or SSB (Escherichia coli).
Quantification of intact substrate and incision products as a percentage of initial substrate as in (A). Error bars represent SEM, n = 3.
A schematic representation of 40 nM XE activity on a “simple fork” in the absence (left) or presence (right) of 80 nM RPA.
Nuclease activity of XE on fork substrates with increasing length of a model nascent leading strand (simple fork; −9; −3; −2; −1; 0 nt from the fork junction) in the presence or absence of 80 nM RPA.
A schematic representation of XE activity in the presence and absence of RPA as in (D).
Nuclease activity of XE on the indicated fork substrates in the presence or absence of RPA. RPA specifically stimulates XE activity on a “simple fork” and “+leading‐strand” substrates.
Fluorescence anisotropy assay to determine the binding constants of RPA for either “simple fork” or “+lagging‐strand” substrates. The blue diamonds denote the fluorophore‐labelled nucleotides. Error bars represent SD, n = 3.
(Top panel) Outline of potential consequences of incubating “+leading‐strand” substrate radiolabelled on the model nascent leading strand with RPA, and the potential products that might be revealed by analysis on a non‐denaturing PAGE gel. (Bottom panel) Nuclease activity performed as in panel a. Reaction products were separated on a 10% non‐denaturing PAGE gel. The DNA substrates remain intact in the RPA alone reactions (lanes 4 and 9), indicating that RPA does not displace the model nascent leading strand or unwind the fork substrates, at the concentrations employed.
Fluorescence anisotropy assay to determine the binding constants of RPA for either “simple fork” or “+leading‐strand” substrates. The blue diamonds denote the fluorophore‐labelled nucleotides.
Nuclease activity of XE on “+leading strand” or DNA:RNA hybrid (5′ ssRNA on the bottom strand) substrates in the presence or absence of 80 nM RPA. RPA cannot stimulate XE to overcome the inhibition of a model nascent leading strand when the 5′‐ssDNA overhang is replaced with 5′ ssRNA. Green line denotes RNA.
(Top panel) Nuclease activity of XE on “+leading‐strand” substrate in the presence or absence of either the WT RPA or the truncated RPA (RPA70C442). (Bottom panel) A schematic representation of the structural domains of WT RPA and RPA70C442. Purple boxes represent the DNA‐binding domains (DBD) designated as A–F. The orange box represents the winged helix domain. RPA70, RPA32 and RPA14 denote the three subunits of RPA.
Limited proteolysis assay to determine structural changes in RPA in the presence or absence of the indicated substrates. 800 nM RPA was incubated with 100 nM unlabelled DNA substrates (simple fork; +leading strand; or no DNA) prior to digestion with 500 nM trypsin in a time course. Reaction samples were separated in Bis‐Tris SDS–PAGE (4–12%) and stained with InstantBlue. Red arrows indicated tryptic digestion pattern of RPA.

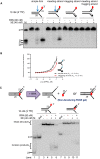
Sequence and schematic structure of a “simple fork” containing a single triazole ICL at the fork junction and its predicted XE nuclease incision products when radiolabelled on the 5′‐end, based on the data obtained on non‐crosslinked fork structure in Figs 1, 2, 3, 4. Green circles denote 5′[32P]‐radiolabelled nucleotides.
(Top panel) Nuclease activity of XE on 3′[32P]‐labelled crosslinked simple fork substrate. (Bottom panel) A schematic representation of the nuclease reaction and the incision products.
(Top panel) Nuclease activity of XE on 5′[32P]‐labelled model native (lanes 2 and 3) and crosslinked (lanes 4 and 5) DNA substrates. The XE incision closest to the fork junction (2 nt from the junction, 26‐mer product) is inhibited in the presence of a crosslink at the fork junction (lane 5). (Bottom panel) Schematic representation of the nuclease reaction and its incision products.
Nuclease activity of XE on 3′[32P]‐radiolabelled crosslinked substrate (simple fork; +leading strand) in the presence or absence of 80 nM RPA. XPF‐ERCC1 incisions reduced by a leading strand are overcome by the presence of RPA (compare lane 5 to lane 6).

(Top panel) Nuclease activity of XE on “simple fork” and +leading‐strand substrates in the presence or absence of RPA and further incubated with 0.8 nM SNM1A in a time course. (Bottom panel) Schematic representation of the nuclease assay reaction products. The blue arrow denotes incision by XE and green Pacman represents digestion by SNM1A. RPA does not affect/alter SNM1A exonuclease activity as seen in the similar stepwise digestion products of SNM1A in the presence or absence of RPA (lanes 2–9). However, the presence of a model leading strand prevents SNM1A from loading onto XE‐RPA‐induced incisions to digest the DNA substrate (lanes 10–17).
(Top panel) Nuclease activity of XE‐RPA on crosslinked DNA substrates (simple fork; +leading strand) and further incubation with SNM1A in a time course. SNM1A digestion inhibition by a model nascent leading strand (as in A) is overcome when an ICL is located at the fork junction.
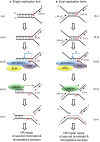
When a single replication fork encounters an ICL, the nascent leading strand initially stalls 20–40 nt from the ICL (“−20” position; step a‐i). It gradually progresses to 1 nt from the ICL (“0” position; step a‐ii), and its arrival at the ICL triggers an XPF‐ERCC1‐RPA‐induced incision six nucleotides 5′ to the junction, in a duplex region (step a‐iii). SNM1A loads from these incisions and digests past the ICL, unhooking the ICL from the DNA duplex, leaving a residual single nucleotide moiety (step a‐iv), which has been demonstrated as the reaction product using mass spectrometry to characterise the reaction products of SNM1A activity in previous work (Wang et al, 2011). This enables translesion synthesis to occur and repair of the broken DNA strand via homologous recombination (step a‐v).
In the event of dual replication fork convergence onto an ICL, both nascent leading strands initially stall ˜20–40 nt from the ICL (step b‐i). CMG complexes from both replication forks unload from both leading strands, as previously described (Long et al, 2014; Zhang et al, 2015) which enables one nascent leading strand to gradually progresses to 1 nt from the ICL (“0” position; step b‐ii) as previously described (Raschle et al, 2008; Zhang et al, 2015). The structure that arises at this stage is inhibitory for XPF‐ERCC1. However, in the presence of RPA, XPF‐ERCC1 will be able to incise the structure (on the lagging‐strand template associated with the fork which has progressed to 0 nt) within the duplex region, 6 nt from the ICL (step b‐iv). This XPF‐ERCC1‐RPA‐induced incision enables SNM1A to load onto and digest past the ICL (step b‐v). The net result of XPF‐ERCC1‐RPA‐SNM1A is ICL unhooking, which enable the translesion (TLS) synthesis step, where the strand extended by the TLS polymerase is the nascent leading strand which remained arrested at ˜20–40 nt from the ICL on the second converged fork and did not strike the ICL (step b‐vi). Homologous recombination‐based repair of the broken chromatid completes repair and facilitates fork restart.
Comment in
-
ERCC1-XPF endonuclease-positioned to cut.EMBO J. 2017 Jul 14;36(14):1993-1995. doi: 10.15252/embj.201797489. Epub 2017 Jun 28. EMBO J. 2017. PMID: 28659377 Free PMC article.
References
-
- Bessho T, Sancar A, Thompson LH, Thelen MP (1997) Reconstitution of human excision nuclease with recombinant XPF‐ERCC1 complex. J Biol Chem 272: 3833–3837 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

