Population Pharmacokinetics of Tigecycline in Critically Ill Patients with Severe Infections
- PMID: 28607024
- PMCID: PMC5527570
- DOI: 10.1128/AAC.00345-17
Population Pharmacokinetics of Tigecycline in Critically Ill Patients with Severe Infections
Abstract
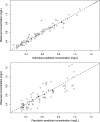
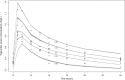
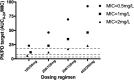
We sought to describe the population pharmacokinetics of tigecycline in critically ill patients and to determine optimized dosing regimens of tigecycline for different bacterial infections. This prospective study included 10 critically ill patients given a standard dose of tigecycline. Blood samples were collected during one dosing interval and were analyzed using validated chromatography. Population pharmacokinetics and Monte Carlo dosing simulations were undertaken using Pmetrics. Three target exposures, expressed as ratios of the 24-h area under the curve to MICs (AUC0-24/MIC), were evaluated (≥17.9 for skin infections, ≥6.96 for intra-abdominal infections, ≥4.5 for hospital-acquired pneumonia). The median age, total body weight, and body mass index (BMI) were 67 years, 69.1 kg, and 24.7 kg/m2, respectively. A two-compartment linear model best described the time course of tigecycline concentrations. The parameter estimates (expressed as means ± standard deviations [SD]) from the final model were as follows: clearance (CL), 7.50 ± 1.11 liters/h; volume in the central compartment, 72.50 ± 21.18 liters; rate constant for tigecycline distribution from the central to the peripheral compartment, 0.31 ± 0.16 h-1; and rate constant for tigecycline distribution from the peripheral to the central compartment, 0.29 ± 0.30 h-1 A larger BMI was associated with increased CL of tigecycline. Licensed doses were found to be sufficient for Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, and methicillin-resistant Staphylococcus aureus for an AUC0-24/MIC target of 4.5 or 6.96. For a therapeutic target of 17.9, an increased tigecycline dose is required, especially for patients with higher BMI. The dosing requirements of tigecycline differ with the indication, with pathogen susceptibility, and potentially with patient BMI.
Keywords: critically ill patients; population pharmacokinetics; severe infections; tigecycline.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Serum and urine pharmacokinetics of tigecycline in obese class III and normal weight adults.J Antimicrob Chemother. 2014 Jan;69(1):190-9. doi: 10.1093/jac/dkt299. Epub 2013 Jul 23. J Antimicrob Chemother. 2014. PMID: 23883872
-
Use of Monte Carlo simulation to evaluate the efficacy of tigecycline and minocycline for the treatment of pneumonia due to carbapenemase-producing Klebsiella pneumoniae.Infect Dis (Lond). 2018 Jul;50(7):507-513. doi: 10.1080/23744235.2018.1423703. Epub 2018 Jan 9. Infect Dis (Lond). 2018. PMID: 29316830
-
Comparison of equal doses of continuous venovenous haemofiltration and haemodiafiltration on ciprofloxacin population pharmacokinetics in critically ill patients.J Antimicrob Chemother. 2016 Jun;71(6):1643-50. doi: 10.1093/jac/dkw043. Epub 2016 Mar 7. J Antimicrob Chemother. 2016. PMID: 26957490
-
Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections.Int J Antimicrob Agents. 2014 Jul;44(1):1-7. doi: 10.1016/j.ijantimicag.2014.01.006. Epub 2014 Feb 6. Int J Antimicrob Agents. 2014. PMID: 24602499 Review.
-
Tigecycline: an update.Diagn Microbiol Infect Dis. 2013 Apr;75(4):331-6. doi: 10.1016/j.diagmicrobio.2012.12.004. Epub 2013 Jan 26. Diagn Microbiol Infect Dis. 2013. PMID: 23357291 Review.
Cited by
-
Effect of pharmacokinetic/pharmacodynamic ratio on tigecycline clinical response and toxicity in critically ill patients with multidrug-resistant Gram-negative infections.SAGE Open Med. 2020 Sep 18;8:2050312120958897. doi: 10.1177/2050312120958897. eCollection 2020. SAGE Open Med. 2020. PMID: 32999720 Free PMC article.
-
Tissue Penetration of Antimicrobials in Intensive Care Unit Patients: A Systematic Review-Part II.Antibiotics (Basel). 2022 Sep 3;11(9):1193. doi: 10.3390/antibiotics11091193. Antibiotics (Basel). 2022. PMID: 36139972 Free PMC article. Review.
-
How to manage KPC infections.Ther Adv Infect Dis. 2020 May 14;7:2049936120912049. doi: 10.1177/2049936120912049. eCollection 2020 Jan-Dec. Ther Adv Infect Dis. 2020. PMID: 32489663 Free PMC article. Review.
-
Optimizing the Dosing Regimens of Tigecycline against Vancomycin-Resistant Enterococci in the Treatment of Intra-abdominal and Skin and Soft Tissue Infections.Infect Chemother. 2020 Sep;52(3):345-351. doi: 10.3947/ic.2020.52.3.345. Infect Chemother. 2020. PMID: 32989939 Free PMC article.
-
Pharmacokinetics of tigecycline in critically ill patients with liver failure defined by maximal liver function capacity test (LiMAx).Ann Intensive Care. 2020 Aug 4;10(1):106. doi: 10.1186/s13613-020-00707-2. Ann Intensive Care. 2020. PMID: 32754775 Free PMC article.
References
-
- Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang Y-C, Maroko RT, Dukart G, Cooper CA. 2010. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 68:140–151. doi:10.1016/j.diagmicrobio.2010.05.012. - DOI - PubMed
-
- Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. 2013. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 57:1756–1762. doi:10.1128/AAC.01232-12. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

