Maintenance of antiangiogenic and antitumor effects by orally active low-dose capecitabine for long-term cancer therapy
- PMID: 28607065
- PMCID: PMC5495268
- DOI: 10.1073/pnas.1705066114
Maintenance of antiangiogenic and antitumor effects by orally active low-dose capecitabine for long-term cancer therapy
Abstract
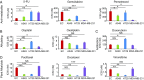
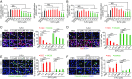
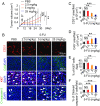
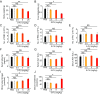
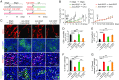
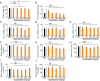
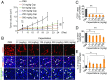
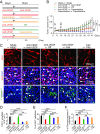
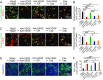
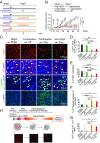
Long-term uninterrupted therapy is essential for maximizing clinical benefits of antiangiogenic drugs (AADs) in cancer patients. Unfortunately, nearly all clinically available AADs are delivered to cancer patients using disrupted regimens. We aim to develop lifetime, nontoxic, effective, orally active, and low-cost antiangiogenic and antitumor drugs for treatment of cancer patients. Here we report our findings of long-term maintenance therapy with orally active, nontoxic, low cost antiangiogenic chemotherapeutics for effective cancer treatment. In a sequential treatment regimen, robust antiangiogenic effects in tumors were achieved with an anti-VEGF drug, followed by a low-dose chemotherapy. The nontoxic, low dose of the orally active prodrug capecitabine was able to sustain the anti-VEGF-induced vessel regression for long periods. In another experimental setting, maintenance of low-dose capecitabine produced greater antiangiogenic and antitumor effects after AAD plus chemotherapy. No obvious adverse effects were developed after more than 2-mo of consecutive treatment with a low dose of capecitabine. Together, our findings provide a rationalized concept of effective cancer therapy by long-term maintenance of AAD-triggered antiangiogenic effects with orally active, nontoxic, low-cost, clinically available chemotherapeutics.
Keywords: VEGF; angiogenesis; cancer therapy; chemotherapy; tumor growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. - PubMed
-
- Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. - PubMed
-
- Wadzinski MG, et al. Heparin-binding angiogenesis factors: Detection by immunological methods. Clin Physiol Biochem. 1987;5:200–209. - PubMed
-
- Davis S, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

