Systems-guided forward genetic screen reveals a critical role of the replication stress response protein ETAA1 in T cell clonal expansion
- PMID: 28607084
- PMCID: PMC5495275
- DOI: 10.1073/pnas.1705795114
Systems-guided forward genetic screen reveals a critical role of the replication stress response protein ETAA1 in T cell clonal expansion
Abstract
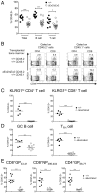
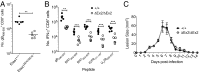
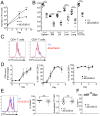
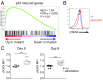
T-cell immunity requires extremely rapid clonal proliferation of rare, antigen-specific T lymphocytes to form effector cells. Here we identify a critical role for ETAA1 in this process by surveying random germ line mutations in mice using exome sequencing and bioinformatic annotation to prioritize mutations in genes of unknown function with potential effects on the immune system, followed by breeding to homozygosity and testing for immune system phenotypes. Effector CD8+ and CD4+ T-cell formation following immunization, lymphocytic choriomeningitis virus (LCMV) infection, or herpes simplex virus 1 (HSV1) infection was profoundly decreased despite normal immune cell development in adult mice homozygous for two different Etaa1 mutations: an exon 2 skipping allele that deletes Gly78-Leu119, and a Cys166Stop truncating allele that eliminates most of the 877-aa protein. ETAA1 deficiency decreased clonal expansion cell autonomously within the responding T cells, causing no decrease in their division rate but increasing TP53-induced mRNAs and phosphorylation of H2AX, a marker of DNA replication stress induced by the ATM and ATR kinases. Homozygous ETAA1-deficient adult mice were otherwise normal, healthy, and fertile, although slightly smaller, and homozygotes were born at lower frequency than expected, consistent with partial lethality after embryonic day 12. Taken together with recently reported evidence in human cancer cell lines that ETAA1 activates ATR kinase through an exon 2-encoded domain, these findings reveal a surprisingly specific requirement for this ATR activator in adult mice restricted to rapidly dividing effector T cells. This specific requirement may provide new ways to suppress pathological T-cell responses in transplantation or autoimmunity.
Keywords: DNA damage; Etaa1; T cell; immunity; replication stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

