Cutting Edge: Active TGF-β1 Released from GARP/TGF-β1 Complexes on the Surface of Stimulated Human B Lymphocytes Increases Class-Switch Recombination and Production of IgA
- PMID: 28607112
- PMCID: PMC5502319
- DOI: 10.4049/jimmunol.1601882
Cutting Edge: Active TGF-β1 Released from GARP/TGF-β1 Complexes on the Surface of Stimulated Human B Lymphocytes Increases Class-Switch Recombination and Production of IgA
Abstract
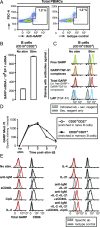
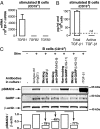
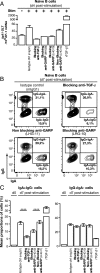
Production of active TGF-β is regulated at a posttranslational level and implies release of the mature cytokine dimer from the inactive, latent TGF-β precursor. There are several cell-type specific mechanisms of TGF-β activation. We identified a new mechanism operating on the surface of human regulatory T cells and involving membrane protein GARP, which binds latent TGF-β1. The paracrine activity of regulatory T cell-derived TGF-β1 contributes to immunosuppression and can be inhibited with anti-GARP Abs. Whether other immune cell types use surface GARP to activate latent TGF-β1 was not known. We show in this study that stimulated, human B lymphocytes produce active TGF-β1 from surface GARP/latent TGF-β1 complexes with isotype switching to IgA production.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures
Similar articles
-
Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin αVβ8.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10161-E10168. doi: 10.1073/pnas.1710680114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109269 Free PMC article.
-
Hepatic Stellate Cells Inhibit T Cells through Active TGF-β1 from a Cell Surface-Bound Latent TGF-β1/GARP Complex.J Immunol. 2015 Sep 15;195(6):2648-56. doi: 10.4049/jimmunol.1500139. Epub 2015 Aug 5. J Immunol. 2015. PMID: 26246140 Free PMC article.
-
Role of GARP in the activation of latent TGF-β1.Mol Biosyst. 2017 Sep 26;13(10):1925-1935. doi: 10.1039/c7mb00251c. Mol Biosyst. 2017. PMID: 28795730 Review.
-
Lysosomal-associated Transmembrane Protein 4B (LAPTM4B) Decreases Transforming Growth Factor β1 (TGF-β1) Production in Human Regulatory T Cells.J Biol Chem. 2015 Aug 14;290(33):20105-16. doi: 10.1074/jbc.M115.655340. Epub 2015 Jun 30. J Biol Chem. 2015. PMID: 26126825 Free PMC article.
-
Garp as a therapeutic target for modulation of T regulatory cell function.Expert Opin Ther Targets. 2017 Feb;21(2):191-200. doi: 10.1080/14728222.2017.1275568. Epub 2016 Dec 29. Expert Opin Ther Targets. 2017. PMID: 28001437 Review.
Cited by
-
New perspectives on the regulation of germinal center reaction via αvβ8- mediated activation of TGFβ.Front Immunol. 2022 Aug 22;13:942468. doi: 10.3389/fimmu.2022.942468. eCollection 2022. Front Immunol. 2022. PMID: 36072589 Free PMC article. Review.
-
Interpreting Immunoregulation in Lung Fibrosis: A New Branch of the Immune Model.Front Immunol. 2021 Aug 20;12:690375. doi: 10.3389/fimmu.2021.690375. eCollection 2021. Front Immunol. 2021. PMID: 34489937 Free PMC article. Review.
-
Glycoprotein A repetitions predominant (GARP) positively regulates transforming growth factor (TGF) β3 and is essential for mouse palatogenesis.J Biol Chem. 2017 Nov 3;292(44):18091-18097. doi: 10.1074/jbc.M117.797613. Epub 2017 Sep 14. J Biol Chem. 2017. PMID: 28912269 Free PMC article.
-
A replication-incompetent CD154/40L recombinant vaccinia virus induces direct and macrophage-mediated antitumor effects in vitro and in vivo.Oncoimmunology. 2019 Feb 14;8(5):e1568162. doi: 10.1080/2162402X.2019.1568162. eCollection 2019. Oncoimmunology. 2019. PMID: 31069131 Free PMC article.
-
B lymphocytes confer immune tolerance via cell surface GARP-TGF-β complex.JCI Insight. 2018 Apr 5;3(7):e99863. doi: 10.1172/jci.insight.99863. eCollection 2018 Apr 5. JCI Insight. 2018. PMID: 29618665 Free PMC article.
References
-
- Marie J. C., Liggitt D., Rudensky A. Y. 2006. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25: 441–454. - PubMed
-
- Munger J. S., Huang X., Kawakatsu H., Griffiths M. J., Dalton S. L., Wu J., Pittet J. F., Kaminski N., Garat C., Matthay M. A., et al. 1999. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

