Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity is Reduced in Preeclampsia
- PMID: 28607124
- PMCID: PMC5507817
- DOI: 10.1161/HYPERTENSIONAHA.117.09321
Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity is Reduced in Preeclampsia
Abstract
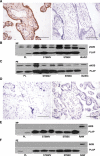
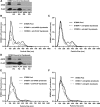
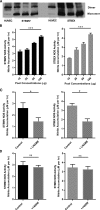
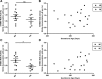
Preeclampsia, a multisystem hypertensive disorder of pregnancy, is associated with increased systemic vascular resistance. Placentae from patients with preeclampsia have reduced levels of endothelial nitric oxide synthase (eNOS) and, thus, less nitric oxide (NO). Syncytiotrophoblast extracellular vesicles (STBEV), comprising microvesicles (STBMV) and exosomes, carry signals from the syncytiotrophoblast to the mother. We hypothesized that STBEV-bound eNOS (STBEV-eNOS), capable of producing NO, are released into the maternal circulation. Dual-lobe ex vivo placental perfusion and differential centrifugation was used to isolate STBEV from preeclampsia (n=8) and normal pregnancies (NP; n=11). Plasma samples of gestational age-matched preeclampsia and NP (n=6) were used to isolate circulating STBMV. STBEV expressed placental alkaline phosphatase, confirming placental origin. STBEV coexpressed eNOS, but not inducible nitric oxide synthase, confirmed using Western blot, flow cytometry, and immunodepletion. STBEV-eNOS produced NO, which was significantly inhibited by N G-nitro-l-arginine methyl ester (eNOS inhibitor; P<0.05) but not by N-(3-(aminomethyl) bezyl) acetamidine) (inducible nitric oxide synthase inhibitor). STBEV-eNOS catalytic activity was confirmed by visualizing eNOS dimerization. STBEV-eNOS was more abundant in uterine vein compared with peripheral blood, indicating placental origin. STBEV isolated from preeclampsia-perfused placentae had lower levels of STBEV-eNOS (STBMV; P<0.05) and overall lower NO activity (STBMV, not significant; syncytiotrophoblast extracellular exosomes, P<0.05) compared with those from NP. Circulating plasma STBMV from preeclampsia women had lower STBEV-eNOS expression compared with that from NP women (P<0.01). This is the first observation of functional eNOS expressed on STBEV from NP and preeclampsia placentae, as well as in plasma. The lower STBEV-eNOS NO production seen in preeclampsia may contribute to the decreased NO bioavailability in this disease.
Keywords: endothelial nitric oxide synthase; hypertension; nitric oxide; preeclampsia; syncytiotrophoblast extracellular vesicles.
© 2017 The Authors.
Figures

Similar articles
-
Syncytiotrophoblast Extracellular Vesicles From Late-Onset Preeclampsia Placentae Suppress Pro-Inflammatory Immune Response in THP-1 Macrophages.Front Immunol. 2021 Jun 7;12:676056. doi: 10.3389/fimmu.2021.676056. eCollection 2021. Front Immunol. 2021. PMID: 34163477 Free PMC article.
-
Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia.Hypertension. 2019 May;73(5):1112-1119. doi: 10.1161/HYPERTENSIONAHA.119.12707. Hypertension. 2019. PMID: 30929513
-
Glycosylated Siglec-6 expression in syncytiotrophoblast-derived extracellular vesicles from preeclampsia placentas.Biochem Biophys Res Commun. 2020 Dec 17;533(4):838-844. doi: 10.1016/j.bbrc.2020.09.081. Epub 2020 Sep 29. Biochem Biophys Res Commun. 2020. PMID: 32998819
-
Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia.J Reprod Immunol. 2017 Feb;119:98-106. doi: 10.1016/j.jri.2016.08.008. Epub 2016 Aug 24. J Reprod Immunol. 2017. PMID: 27613663 Review.
-
Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia.Redox Biol. 2021 Apr;40:101861. doi: 10.1016/j.redox.2021.101861. Epub 2021 Jan 19. Redox Biol. 2021. PMID: 33548859 Free PMC article. Review.
Cited by
-
Early prediction of pre-eclampsia using circulating placental exosomes: Newer insights.Indian J Med Res. 2023 Oct 1;158(4):385-396. doi: 10.4103/ijmr.ijmr_2143_22. Epub 2023 Sep 25. Indian J Med Res. 2023. PMID: 37987999 Free PMC article. Review.
-
Non-Coding RNAs in Preeclampsia-Molecular Mechanisms and Diagnostic Potential.Int J Mol Sci. 2021 Sep 30;22(19):10652. doi: 10.3390/ijms221910652. Int J Mol Sci. 2021. PMID: 34638993 Free PMC article. Review.
-
Extracellular Vesicles: An Important Biomarker in Recurrent Pregnancy Loss?J Clin Med. 2021 Jun 9;10(12):2549. doi: 10.3390/jcm10122549. J Clin Med. 2021. PMID: 34207656 Free PMC article.
-
In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology.J Extracell Vesicles. 2022 Jan;11(1):e12151. doi: 10.1002/jev2.12151. J Extracell Vesicles. 2022. PMID: 35041249 Free PMC article. Review.
-
Molecular Targets of Aspirin and Prevention of Preeclampsia and Their Potential Association with Circulating Extracellular Vesicles during Pregnancy.Int J Mol Sci. 2019 Sep 5;20(18):4370. doi: 10.3390/ijms20184370. Int J Mol Sci. 2019. PMID: 31492014 Free PMC article. Review.
References
-
- Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. - PubMed
-
- WHO. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. World Health Organization; 2011. - PubMed
-
- Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1451. - PubMed
-
- Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia—two placental causes of preeclampsia? Placenta. 2014;35(suppl):S20–S25. doi: 10.1016/j.placenta.2013.12.008. - PubMed
-
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

