Unique versus Redundant Functions of Neuroligin Genes in Shaping Excitatory and Inhibitory Synapse Properties
- PMID: 28607166
- PMCID: PMC5518416
- DOI: 10.1523/JNEUROSCI.0125-17.2017
Unique versus Redundant Functions of Neuroligin Genes in Shaping Excitatory and Inhibitory Synapse Properties
Abstract
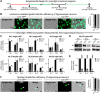
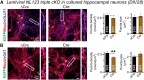
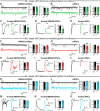
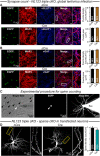
Neuroligins are evolutionarily conserved postsynaptic cell adhesion molecules that interact with presynaptic neurexins. Neurons express multiple neuroligin isoforms that are targeted to specific synapses, but their synaptic functions and mechanistic redundancy are not completely understood. Overexpression or RNAi-mediated knockdown of neuroligins, respectively, causes a dramatic increase or decrease in synapse density, whereas genetic deletions of neuroligins impair synapse function with only minor effects on synapse numbers, raising fundamental questions about the overall physiological role of neuroligins. Here, we have systematically analyzed the effects of conditional genetic deletions of all major neuroligin isoforms (i.e., NL1, NL2, and NL3), either individually or in combinations, in cultured mouse hippocampal and cortical neurons. We found that conditional genetic deletions of neuroligins caused no change or only a small change in synapses numbers, but strongly impaired synapse function. This impairment was isoform specific, suggesting that neuroligins are not functionally redundant. Sparse neuroligin deletions produced phenotypes comparable to those of global deletions, indicating that neuroligins function in a cell-autonomous manner. Mechanistically, neuroligin deletions decreased the synaptic levels of neurotransmitter receptors and had no effect on presynaptic release probabilities. Overexpression of neuroligin-1 in control or neuroligin-deficient neurons increased synaptic transmission and synapse density but not spine numbers, suggesting that these effects reflect a gain-of-function mechanism; whereas overexpression of neuroligin-3, which, like neuroligin-1 is also targeted to excitatory synapses, had no comparable effect. Our data demonstrate that neuroligins are required for the physiological organization of neurotransmitter receptors in postsynaptic specializations and suggest that they do not play a major role in synapse formation.SIGNIFICANCE STATEMENT Human neuroligin genes have been associated with autism, but the cellular functions of different neuroligins and their molecular mechanisms remain incompletely understood. Here, we performed comparative analyses in cultured mouse neurons of all major neuroligin isoforms, either individually or in combinations, using conditional knockouts. We found that neuroligin deletions did not affect synapse numbers but differentially impaired excitatory or inhibitory synaptic functions in an isoform-specific manner. These impairments were due, at least in part, to a decrease in synaptic distribution of neurotransmitter receptors upon deletion of neuroligins. Conversely, the overexpression of neuroligin-1 increased synapse numbers but not spine numbers. Our results suggest that various neuroligin isoforms perform unique postsynaptic functions in organizing synapses but are not essential for synapse formation or maintenance.
Keywords: conditional knockout; neuroligin; primary neuronal culture; synapse development; synaptic transmission; synaptogenesis.
Copyright © 2017 the authors 0270-6474/17/376816-21$15.00/0.
Figures










Comment in
-
Refining the Roles of Neuroligins in Synapse Development and Function: A Reductionist Conditional Knock-out Approach.J Neurosci. 2017 Dec 6;37(49):11769-11771. doi: 10.1523/JNEUROSCI.2492-17.2017. J Neurosci. 2017. PMID: 29212945 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
