Exogenous interleukin 37 ameliorates atherosclerosis via inducing the Treg response in ApoE-deficient mice
- PMID: 28607385
- PMCID: PMC5468328
- DOI: 10.1038/s41598-017-02987-4
Exogenous interleukin 37 ameliorates atherosclerosis via inducing the Treg response in ApoE-deficient mice
Abstract
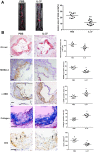
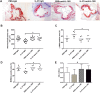
Our previous study indicated that interleukin (IL)-37 is involved in atherosclerosis. In the present study, Anterior tibial arteries were collected from diabetes patients and controls. A histopathological analysis showed that IL-37 was over-expressed in human atherosclerotic plaques. Many types of cells including macrophages, vascular smooth muscle cells (VSMCs), endothelial cells and T lymphocyte expressed IL-37 in human atherosclerotic plaques. ApoE-/- mice were divided into a control group and a recombinant human IL-37-treated group. The IL-37 treatment resulted in a significant decrease in macrophages and CD4+ T lymphocytes and a substantial increase in VSMCs and collagen in atherosclerotic plaques, resulting in a reduction in atherosclerotic plaque size. Furthermore, the IL-37 treatment modulated the CD4+ T lymphocyte activity, including a decrease in T helper cell type 1 (Th1) and Th17 cells and an increase in regulatory T (Treg) cells, and inhibited the maturity of dendritic cells both in vivo and in vitro. In addition, treatment with anti-IL-10 receptor monoclonal antibody abrogated the anti-atherosclerotic effects of IL-37. These data suggest that exogenous IL-37 ameliorates atherosclerosis via inducing the Treg response. IL-37 may be a novel therapeutic to prevent and treat atherosclerotic disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
TSLPR deficiency attenuates atherosclerotic lesion development associated with the inhibition of TH17 cells and the promotion of regulator T cells in ApoE-deficient mice.J Mol Cell Cardiol. 2014 Nov;76:33-45. doi: 10.1016/j.yjmcc.2014.07.003. Epub 2014 Aug 10. J Mol Cell Cardiol. 2014. PMID: 25117469
-
Transgenic Overexpression of IL-37 Protects Against Atherosclerosis and Strengthens Plaque Stability.Cell Physiol Biochem. 2018;45(3):1034-1050. doi: 10.1159/000487344. Epub 2018 Feb 7. Cell Physiol Biochem. 2018. PMID: 29439249
-
Smooth Muscle Cell-Derived Interleukin-17C Plays an Atherogenic Role via the Recruitment of Proinflammatory Interleukin-17A+ T Cells to the Aorta.Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1496-506. doi: 10.1161/ATVBAHA.116.307892. Epub 2016 Jun 30. Arterioscler Thromb Vasc Biol. 2016. PMID: 27365405 Free PMC article.
-
Regulatory T cells as a new therapeutic target for atherosclerosis.Acta Pharmacol Sin. 2018 Aug;39(8):1249-1258. doi: 10.1038/aps.2017.140. Epub 2018 Jan 11. Acta Pharmacol Sin. 2018. PMID: 29323337 Free PMC article. Review.
-
Treg cells in atherosclerosis.Mol Biol Rep. 2021 May;48(5):4897-4910. doi: 10.1007/s11033-021-06483-x. Epub 2021 Jun 12. Mol Biol Rep. 2021. PMID: 34117978 Review.
Cited by
-
Circulating IL-37 levels are elevated in patients with hypertension.Exp Ther Med. 2021 Jun;21(6):558. doi: 10.3892/etm.2021.9990. Epub 2021 Mar 26. Exp Ther Med. 2021. PMID: 33850530 Free PMC article.
-
Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease.Circ Res. 2020 Apr 24;126(9):1260-1280. doi: 10.1161/CIRCRESAHA.120.315937. Epub 2020 Apr 23. Circ Res. 2020. PMID: 32324502 Free PMC article. Review.
-
Exogenous Interleukin-37 Alleviates Hepatitis with Reduced Dendritic Cells and Induced Regulatory T Cells in Acute Murine Cytomegalovirus Infection.J Immunol Res. 2023 May 12;2023:1462048. doi: 10.1155/2023/1462048. eCollection 2023. J Immunol Res. 2023. PMID: 37215069 Free PMC article.
-
The Effects of Selective Hematopoietic Expression of Human IL-37 on Systemic Inflammation and Atherosclerosis in LDLr-Deficient Mice.Int J Mol Sci. 2017 Aug 9;18(8):1672. doi: 10.3390/ijms18081672. Int J Mol Sci. 2017. PMID: 28792474 Free PMC article.
-
The IL-1 Family and Its Role in Atherosclerosis.Int J Mol Sci. 2022 Dec 20;24(1):17. doi: 10.3390/ijms24010017. Int J Mol Sci. 2022. PMID: 36613465 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

