Early Gut Microbiota Intervention Suppresses DSS-Induced Inflammatory Responses by Deactivating TLR/NLR Signalling in Pigs
- PMID: 28607413
- PMCID: PMC5468271
- DOI: 10.1038/s41598-017-03161-6
Early Gut Microbiota Intervention Suppresses DSS-Induced Inflammatory Responses by Deactivating TLR/NLR Signalling in Pigs
Abstract
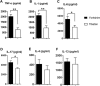
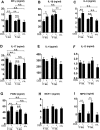
Recent metagenomic studies suggest that innate and adaptive immune phenotypes can be programmed via gut microbiota-host interactions mediated via activation of pattern recognition receptors (PRRs) on host cells. In this study, we used two extremely different pig lines (the Yorkshire and the Tibetan) to test the hypothesis that the transplantation of gut microbiota could transfer certain immunologic characteristics from donor to recipient. The faecal microbiota of these two pig lines was transplanted in healthy commercial hybrid newborn piglets to establish the "Tibetan-intervened" and "Yorkshire-intervened" porcine models. Then, acute colitis was induced using dextran sulphate sodium (DSS), which activated Toll-/NOD-like receptor (TLR/NLR) signalling in the colonic tissues of the "Yorkshire-intervened" piglets, leading to increases in pro-inflammatory cytokines and immune cells and causing intestinal injuries. Conversely, DSS administration had little influence on the "Tibetan-intervened" piglets, which showed no significant inflammation and no changes in cytokines, immune cells, or signalling molecules, including TLRs, NLRs, MYD88 and NF-κB, after DSS treatment. These results indicate that pigs inoculated with the Tibetan microbiota acquired relatively strong resistance to experimental colitis, suggesting that the genotype of the host contributes to the uniqueness of its intestinal microbial community, whereas the microbiota plays a vital role in programming the immune phenotypes of the host.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice.Gut. 2016 Jun;65(6):935-43. doi: 10.1136/gutjnl-2014-308323. Epub 2015 Mar 11. Gut. 2016. PMID: 25761602 Free PMC article.
-
Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis.Gastroenterology. 2018 Mar;154(4):1009-1023.e14. doi: 10.1053/j.gastro.2017.11.003. Epub 2017 Nov 11. Gastroenterology. 2018. PMID: 29133078
-
Toll-Like Receptor 7 Agonist-Induced Dermatitis Causes Severe Dextran Sulfate Sodium Colitis by Altering the Gut Microbiome and Immune Cells.Cell Mol Gastroenterol Hepatol. 2018 Sep 25;7(1):135-156. doi: 10.1016/j.jcmgh.2018.09.010. eCollection 2019. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30510995 Free PMC article.
-
Gut microbiota, NLR proteins, and intestinal homeostasis.J Exp Med. 2020 Oct 5;217(10):e20181832. doi: 10.1084/jem.20181832. J Exp Med. 2020. PMID: 32941596 Free PMC article. Review.
-
Crosstalk Between The Immune Receptors and Gut Microbiota.Curr Protein Pept Sci. 2015;16(7):622-31. doi: 10.2174/1389203716666150630134356. Curr Protein Pept Sci. 2015. PMID: 26122782 Review.
Cited by
-
Dynamic distribution of gut microbiota in meat rabbits at different growth stages and relationship with average daily gain (ADG).BMC Microbiol. 2020 May 14;20(1):116. doi: 10.1186/s12866-020-01797-5. BMC Microbiol. 2020. PMID: 32410629 Free PMC article.
-
4,4'-Diaminodiphenyl Sulfone (DDS) as an Inflammasome Competitor.Int J Mol Sci. 2020 Aug 19;21(17):5953. doi: 10.3390/ijms21175953. Int J Mol Sci. 2020. PMID: 32824985 Free PMC article.
-
Dietary nutrition, intestinal microbiota dysbiosis and post-weaning diarrhea in piglets.Anim Nutr. 2024 Feb 28;17:188-207. doi: 10.1016/j.aninu.2023.12.010. eCollection 2024 Jun. Anim Nutr. 2024. PMID: 38800735 Free PMC article. Review.
-
Gut microbiome and resistome characterization of pigs treated with commonly used post-weaning diarrhea treatments.Anim Microbiome. 2024 May 3;6(1):24. doi: 10.1186/s42523-024-00307-6. Anim Microbiome. 2024. PMID: 38702766 Free PMC article.
-
Prevotella in Pigs: The Positive and Negative Associations with Production and Health.Microorganisms. 2020 Oct 14;8(10):1584. doi: 10.3390/microorganisms8101584. Microorganisms. 2020. PMID: 33066697 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

