Benzo(a)pyrene triggers desensitization of β2-adrenergic pathway
- PMID: 28607424
- PMCID: PMC5468268
- DOI: 10.1038/s41598-017-03646-4
Benzo(a)pyrene triggers desensitization of β2-adrenergic pathway
Abstract
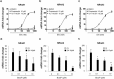
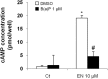
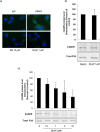
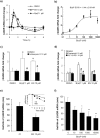
Exposure to environmental polycyclic aromatic hydrocarbons (PAHs), such as benzo(a)pyrene (B(a)P), has been linked to several health-threatening risks. PAHs were also shown to hinder adrenergic receptor (ADR) responses. As we previously demonstrated that B(a)P can directly interact with the β2ADR, we investigated here whether B(a)P could decrease β2ADR responsiveness by triggering receptor desensitization phenomena. We firstly showed that exposure to B(a)P reduced β2ADR-mediated epinephrine-induced induction of NR4A gene mRNAs and of intracellular cAMP. Analysis of β2ADR protein expression demonstrated that B(a)P rapidly decreased membrane expression of β2ADR with a subsequent degradation of receptor protein. B(a)P exposure concomitantly rapidly increased the β2ADR mRNA levels. The use of the β-blockers, propranolol and ICI 118.551, demonstrated the involvement of β2ADR itself in this increase. However, sustained exposure to B(a)P induced a diminution of β2ADR mRNA steady-state as a result of the acceleration of its degradation. Together, these results show that, beside the well-known activation of the aryl hydrocarbon receptor, PAH deleterious effects may involve the dysfunction of adrenergic responses through, in part, the desensitization of β2ADR. This may be taken in consideration when β2-agonists/antagonists are administered in patients exposed to important concentrations of PAHs, e.g. in cigarette smokers.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Induction of intracellular calcium concentration by environmental benzo(a)pyrene involves a β2-adrenergic receptor/adenylyl cyclase/Epac-1/inositol 1,4,5-trisphosphate pathway in endothelial cells.J Biol Chem. 2012 Feb 3;287(6):4041-52. doi: 10.1074/jbc.M111.319970. Epub 2011 Dec 13. J Biol Chem. 2012. PMID: 22167199 Free PMC article.
-
An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation.Endocrinology. 2007 Jul;148(7):3441-8. doi: 10.1210/en.2006-1646. Epub 2007 Apr 19. Endocrinology. 2007. PMID: 17446185
-
β2-adrenergic receptor-mediated in vitro regulation of human hepatic drug transporter expression by epinephrine.Eur J Pharm Sci. 2017 Aug 30;106:302-312. doi: 10.1016/j.ejps.2017.06.010. Epub 2017 Jun 8. Eur J Pharm Sci. 2017. PMID: 28603032
-
Effects of milrinone on contractility and cyclic adenosine monophosphate production induced by beta1- and beta2-adrenergic receptor activation in human myocardium.Clin Ther. 2007 Aug;29(8):1718-24. doi: 10.1016/j.clinthera.2007.08.009. Clin Ther. 2007. PMID: 17919552
-
Differential adrenergic regulation of the gene expression of the beta-adrenoceptor subtypes beta1, beta2 and beta3 in brown adipocytes.Biochem J. 2000 May 1;347 Pt 3(Pt 3):643-51. Biochem J. 2000. PMID: 10769166 Free PMC article.
Cited by
-
Potential role of polycyclic aromatic hydrocarbons as mediators of cardiovascular effects from combustion particles.Environ Health. 2019 Aug 22;18(1):74. doi: 10.1186/s12940-019-0514-2. Environ Health. 2019. PMID: 31439044 Free PMC article. Review.
-
The β2-adrenergic biased agonist nebivolol inhibits the development of Th17 and the response of memory Th17 cells in an NF-κB-dependent manner.Front Immunol. 2024 Oct 9;15:1446424. doi: 10.3389/fimmu.2024.1446424. eCollection 2024. Front Immunol. 2024. PMID: 39445009 Free PMC article.
-
Effects of organic chemicals from diesel exhaust particles on adipocytes differentiated from human mesenchymal stem cells.Basic Clin Pharmacol Toxicol. 2023 Jan;132(1):83-97. doi: 10.1111/bcpt.13805. Epub 2022 Oct 18. Basic Clin Pharmacol Toxicol. 2023. PMID: 36214226 Free PMC article.
-
Genome-Wide Transcriptional and Functional Analysis of Human T Lymphocytes Treated with Benzo[α]pyrene.Int J Mol Sci. 2018 Nov 17;19(11):3626. doi: 10.3390/ijms19113626. Int J Mol Sci. 2018. PMID: 30453624 Free PMC article.
-
Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases.Respir Res. 2020 Nov 13;21(1):299. doi: 10.1186/s12931-020-01563-1. Respir Res. 2020. PMID: 33187512 Free PMC article. Review.
References
-
- Skupińska K, Misiewicz I, Kasprzycka-Guttman T. Polycyclic aromatic hydrocarbons: physicochemical properties, environmental appearance and impact on living organisms. Acta Pol. Pharm. 2004;61:233–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

