A translational approach to capture gait signatures of neurological disorders in mice and humans
- PMID: 28607434
- PMCID: PMC5468293
- DOI: 10.1038/s41598-017-03336-1
A translational approach to capture gait signatures of neurological disorders in mice and humans
Abstract
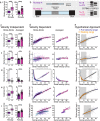
A method for capturing gait signatures in neurological conditions that allows comparison of human gait with animal models would be of great value in translational research. However, the velocity dependence of gait parameters and differences between quadruped and biped gait have made this comparison challenging. Here we present an approach that accounts for changes in velocity during walking and allows for translation across species. In mice, we represented spatial and temporal gait parameters as a function of velocity and established regression models that reproducibly capture the signatures of these relationships during walking. In experimental parkinsonism models, regression curves representing these relationships shifted from baseline, implicating changes in gait signatures, but with marked differences between models. Gait parameters in healthy human subjects followed similar strict velocity dependent relationships which were altered in Parkinson's patients in ways that resemble some but not all mouse models. This novel approach is suitable to quantify qualitative walking abnormalities related to CNS circuit dysfunction across species, identify appropriate animal models, and it provides important translational opportunities.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

