Geographic variation in pneumococcal vaccine efficacy estimated from dynamic modeling of epidemiological data post-PCV7
- PMID: 28607461
- PMCID: PMC5468270
- DOI: 10.1038/s41598-017-02955-y
Geographic variation in pneumococcal vaccine efficacy estimated from dynamic modeling of epidemiological data post-PCV7
Abstract
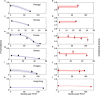
Although mean efficacy of multivalent pneumococcus vaccines has been intensively studied, variance in vaccine efficacy (VE) has been overlooked. Different net individual protection across settings can be driven by environmental conditions, local serotype and clonal composition, as well as by socio-demographic and genetic host factors. Understanding efficacy variation has implications for population-level effectiveness and other eco-evolutionary feedbacks. Here I show that realized VE can vary across epidemiological settings, by applying a multi-site-one-model approach to data post-vaccination. I analyse serotype prevalence dynamics following PCV7, in asymptomatic carriage in children attending day care in Portugal, Norway, France, Greece, Hungary and Hong-Kong. Model fitting to each dataset provides site-specific estimates for vaccine efficacy against acquisition, and pneumococcal transmission parameters. According to this model, variable serotype replacement across sites can be explained through variable PCV7 efficacy, ranging from 40% in Norway to 10% in Hong-Kong. While the details of how this effect is achieved remain to be determined, here I report three factors negatively associated with the VE readout, including initial prevalence of serotype 19F, daily mean temperature, and the Gini index. The study warrants more attention on local modulators of vaccine performance and calls for predictive frameworks within and across populations.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Dagan R, et al. Serum serotype-specific pneumococcal anticapsular immunoglobulin g concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. Journal of Infectious Diseases. 2005;192:367–376. doi: 10.1086/431679. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

