Clostridium difficile flagella induce a pro-inflammatory response in intestinal epithelium of mice in cooperation with toxins
- PMID: 28607468
- PMCID: PMC5468286
- DOI: 10.1038/s41598-017-03621-z
Clostridium difficile flagella induce a pro-inflammatory response in intestinal epithelium of mice in cooperation with toxins
Abstract
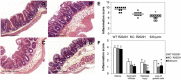
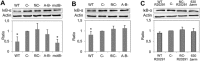
Clostridium difficile is the most important enteropathogen involved in gut nosocomial post-antibiotic infections. The emergence of hypervirulent strains has contributed to increased mortality and morbidity of CDI. The C. difficile toxins contribute directly to CDI-associated lesions of the gut, but other bacterial factors are needed for the bacteria to adhere and colonize the intestinal epithelium. The C. difficile flagella, which confer motility and chemotaxis for successful intestinal colonization, could play an additional role in bacterial pathogenesis by contributing to the inflammatory response of the host and mucosal injury. Indeed, by activating the TLR5, flagella can elicit activation of the MAPK and NF-κB cascades of cell signaling, leading to the secretion of pro-inflammatory cytokines. In the current study, we demonstrate, by using an animal model of CDI, a synergic effect of flagella and toxins in eliciting an inflammatory mucosal response. In this model, the absence of flagella dramatically decreases the degree of mucosal inflammation in mice and the sole presence of toxins without flagella was not enough to elicit epithelial lesions. These results highlight the important role of C. difficile flagella in eliciting mucosal lesions as long as the toxins exert their action on the epithelium.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Cloud J, Kelly CP. Update on Clostridium difficile associated disease. Curr Opin Gastroenterol. 2007;23:4–9. - PubMed
-
- Cohen SH, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

