Precocious centriole disengagement and centrosome fragmentation induced by mitotic delay
- PMID: 28607478
- PMCID: PMC5474744
- DOI: 10.1038/ncomms15803
Precocious centriole disengagement and centrosome fragmentation induced by mitotic delay
Abstract
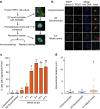
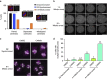
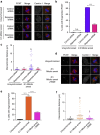
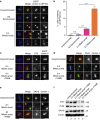
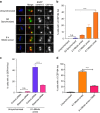
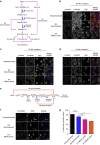
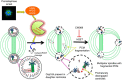
The spindle assembly checkpoint (SAC) delays mitotic progression until all sister chromatid pairs achieve bi-orientation, and while the SAC can maintain mitotic arrest for extended periods, moderate delays in mitotic progression have significant effects on the resulting daughter cells. Here we show that when retinal-pigmented epithelial (RPE1) cells experience mitotic delay, there is a time-dependent increase in centrosome fragmentation and centriole disengagement. While most cells with disengaged centrioles maintain spindle bipolarity, clustering of disengaged centrioles requires the kinesin-14, HSET. Centrosome fragmentation and precocious centriole disengagement depend on separase and anaphase-promoting complex/cyclosome (APC/C) activity, which also triggers the acquisition of distal appendage markers on daughter centrioles and the loss of procentriolar markers. Together, these results suggest that moderate delays in mitotic progression trigger the initiation of centriole licensing through centriole disengagement, at which point the ability to maintain spindle bipolarity becomes a function of HSET-mediated spindle pole clustering.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Mechanism limiting centrosome duplication to once per cell cycle.Nature. 2006 Aug 24;442(7105):947-51. doi: 10.1038/nature04985. Epub 2006 Jul 19. Nature. 2006. PMID: 16862117
-
Oscillation of APC/C activity during cell cycle arrest promotes centrosome amplification.J Cell Sci. 2012 Nov 15;125(Pt 22):5353-68. doi: 10.1242/jcs.106096. Epub 2012 Sep 6. J Cell Sci. 2012. PMID: 22956538 Free PMC article.
-
PCNT is critical for the association and conversion of centrioles to centrosomes during mitosis.J Cell Sci. 2019 Mar 26;132(6):jcs225789. doi: 10.1242/jcs.225789. J Cell Sci. 2019. PMID: 30814333
-
Centriole inheritance.Prion. 2008 Jan-Mar;2(1):9-16. doi: 10.4161/pri.2.1.5064. Epub 2008 Jan 12. Prion. 2008. PMID: 19164929 Free PMC article. Review.
-
Do centrioles generate a polar ejection force?Riv Biol. 2005 Jan-Apr;98(1):71-95. Riv Biol. 2005. PMID: 15889341 Review.
Cited by
-
Orbit/CLASP determines centriole length by antagonising Klp10A in Drosophila spermatocytes.J Cell Sci. 2021 Mar 26;134(6):jcs251231. doi: 10.1242/jcs.251231. J Cell Sci. 2021. PMID: 33674447 Free PMC article.
-
Mild replication stress causes chromosome mis-segregation via premature centriole disengagement.Nat Commun. 2019 Aug 8;10(1):3585. doi: 10.1038/s41467-019-11584-0. Nat Commun. 2019. PMID: 31395887 Free PMC article.
-
MLL/WDR5 complex recruits centriolar satellite protein Cep72 to regulate microtubule nucleation and spindle formation.Sci Adv. 2024 Dec 13;10(50):eadn0086. doi: 10.1126/sciadv.adn0086. Epub 2024 Dec 11. Sci Adv. 2024. PMID: 39661677 Free PMC article.
-
Distinct dynein complexes defined by DYNLRB1 and DYNLRB2 regulate mitotic and male meiotic spindle bipolarity.Nat Commun. 2023 Mar 27;14(1):1715. doi: 10.1038/s41467-023-37370-7. Nat Commun. 2023. PMID: 36973253 Free PMC article.
-
Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis.Cells. 2022 Apr 16;11(8):1360. doi: 10.3390/cells11081360. Cells. 2022. PMID: 35456039 Free PMC article.
References
-
- Musacchio A. & Hardwick K. G. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol. 3, 731–741 (2002). - PubMed
-
- Gascoigne K. E. & Taylor S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

