Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet?
- PMID: 28607506
- PMCID: PMC5637230
- DOI: 10.1038/tpj.2017.21
Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet?
Abstract
Pharmacogenetics (PGx) has the potential to personalize pharmaceutical treatments. Many relevant gene-drug associations have been discovered, but PGx-guided treatment needs to be cost-effective as well as clinically beneficial to be incorporated into standard health-care. We reviewed economic evaluations for PGx associations listed in the US Food and Drug Administration (FDA) Table of Pharmacogenomic Biomarkers in Drug Labeling. We determined the proportion of evaluations that found PGx-guided treatment to be cost-effective or dominant over the alternative strategies, and estimated the impact on this proportion of removing the cost of genetic testing. Of the 137 PGx associations in the FDA table, 44 economic evaluations, relating to 10 drugs, were identified. Of these evaluations, 57% drew conclusions in favour of PGx testing, of which 30% were cost-effective and 27% were dominant (cost-saving). If genetic information was freely available, 75% of economic evaluations would support PGx-guided treatment, of which 25% would be cost-effective and 50% would be dominant. Thus, PGx-guided treatment can be a cost-effective and even a cost-saving strategy. Having genetic information readily available in the clinical health record is a realistic future prospect, and would make more genetic tests economically worthwhile.
Conflict of interest statement
MV is funded by a studentship from the Medical Research Council and Eli Lilly and Company Ltd. MEW is a part-time employee of Genomics plc. CML declares no conflict of interest.
Figures


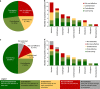
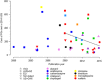
References
-
- Mallal S, Phillips E, Carosi G, Molina J-M, Workman C, Tomažič J et al. HLA-B* 5701 screening for hypersensitivity to abacavir. N Engl J Med 2008; 358: 568–579. - PubMed
-
- Chung W-H, Hung S-I, Hong H-S, Hsih M-S, Yang L-C, Ho H-C et al. Medical genetics: a marker for Stevens–Johnson syndrome. Nature 2004; 428: 486–486. - PubMed
-
- U.S. Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. Available at http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/... (accessed on 18 September 2015).
-
- Centers for Disease Control and Prevention. Genomic Tests and Family Health History by Levels of Evidence: Public Health Genomics. Available at http://www.cdc.gov/genomics/gtesting/tier.htm (accessed on 17 February 2016).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

