Synthesis and Optical Properties of Pentamethine Cyanine Dyes With Carboxylic Acid Moieties
- PMID: 28607539
- PMCID: PMC5457140
- DOI: 10.1177/1177390117711938
Synthesis and Optical Properties of Pentamethine Cyanine Dyes With Carboxylic Acid Moieties
Abstract
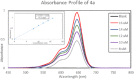
Cyanine dyes possessing carboxylic acid groups have been used in many different fields of study. The acid groups can act as handles for bioconjugation or as metal chelators. Several pentamethine cyanine dyes with propionic acid handles were synthesized and their optical properties were studied to determine their usefulness as fluorescent probes. The optical properties studies performed include the absorbance and emission maxima values as well as the calculation of quantum yield and molecular brightness levels. Molecular models were also calculated to help analyze the dyes' behavior and were compared with similar dyes with varying alkyl chain lengths replacing the acid moieties.
Keywords: Carboxylic acid; cyanine; optical properties; pentamethine.
Conflict of interest statement
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Disclosures and Ethics As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Figures



Similar articles
-
Far-red pentamethine cyanine dyes as fluorescent probes for the detection of serum albumins.R Soc Open Sci. 2020 Jul 1;7(7):200453. doi: 10.1098/rsos.200453. eCollection 2020 Jul. R Soc Open Sci. 2020. PMID: 32874638 Free PMC article.
-
The effect of varying short-chain alkyl substitution on the molar absorptivity and quantum yield of cyanine dyes.Anal Chem Insights. 2011;6:29-36. doi: 10.4137/ACI.S6568. Epub 2011 Mar 23. Anal Chem Insights. 2011. PMID: 21760707 Free PMC article.
-
Shortwave Infrared Absorptive and Emissive Pentamethine-Bridged Indolizine Cyanine Dyes.J Org Chem. 2021 Nov 5;86(21):15376-15386. doi: 10.1021/acs.joc.1c01908. Epub 2021 Oct 14. J Org Chem. 2021. PMID: 34647452
-
Potential of Cyanine Derived Dyes in Photodynamic Therapy.Pharmaceutics. 2021 May 31;13(6):818. doi: 10.3390/pharmaceutics13060818. Pharmaceutics. 2021. PMID: 34072719 Free PMC article. Review.
-
Cyanine Dyes Containing Quinoline Moieties: History, Synthesis, Optical Properties, and Applications.Chemistry. 2021 Mar 1;27(13):4230-4248. doi: 10.1002/chem.202003697. Epub 2020 Dec 29. Chemistry. 2021. PMID: 33137212 Free PMC article. Review.
Cited by
-
Synergetic effects of Cu/TiO2 sensitized with different cyanine dyes on hydrogen evolution.RSC Adv. 2019 Aug 8;9(43):24670-24681. doi: 10.1039/c9ra04696h. eCollection 2019 Aug 8. RSC Adv. 2019. PMID: 35528639 Free PMC article.
-
Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges.Bioconjug Chem. 2020 Feb 19;31(2):194-213. doi: 10.1021/acs.bioconjchem.9b00482. Epub 2019 Aug 12. Bioconjug Chem. 2020. PMID: 31365819 Free PMC article. Review.
-
Synthesis and Optical Properties of Near-Infrared meso-Phenyl-Substituted Symmetric Heptamethine Cyanine Dyes.Molecules. 2018 Jan 24;23(2):226. doi: 10.3390/molecules23020226. Molecules. 2018. PMID: 29364846 Free PMC article.
-
Near-Infrared Heptamethine Cyanine Dyes for Nanoparticle-Based Photoacoustic Imaging and Photothermal Therapy.J Med Chem. 2021 Jun 24;64(12):8798-8805. doi: 10.1021/acs.jmedchem.1c00771. Epub 2021 Jun 3. J Med Chem. 2021. PMID: 34081463 Free PMC article.
-
Counter Anion Effect on the Photophysical Properties of Emissive Indolizine-Cyanine Dyes in Solution and Solid State.Molecules. 2018 Nov 22;23(12):3051. doi: 10.3390/molecules23123051. Molecules. 2018. PMID: 30469460 Free PMC article.
References
-
- Mishra A, Behera RK, Behera PK, Mishra BK, Behera GB. Cyanines during the 1990s: a review. Chem Rev. 2000;100:1973–2012. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

