Molecular mechanisms underlying the pilsicainide-induced stabilization of hERG proteins in transfected mammalian cells
- PMID: 28607619
- PMCID: PMC5459418
- DOI: 10.1016/j.joa.2016.09.003
Molecular mechanisms underlying the pilsicainide-induced stabilization of hERG proteins in transfected mammalian cells
Abstract
Background: Pilsicainide, classified as a relatively selective Na+ channel blocker, also has an inhibitory action on the rapidly-activating delayed-rectifier K+ current (IKr ) through human ether-a-go-go-related gene (hERG) channels. We studied the effects of chronic exposure to pilsicainide on the expression of wild-type (WT) hERG proteins and WT-hERG channel currents, as well as on the expression of mutant hERG proteins, in a heterologous expression system.
Methods: HEK293 cells stably expressing WT or mutant hERG proteins were subjected to Western blotting, immunofluorescence microscopy and patch-clamp experiments.
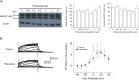
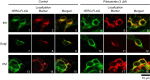
Results: Acute exposure to pilsicainide at 0.03-10 μM influenced neither the expression of WT-hERG proteins nor WT-hERG channel currents. Chronic treatment with 0.03-10 μM pilsicainide for 48 h, however, increased the expression of WT-hERG proteins and channel currents in a concentration-dependent manner. Chronic treatment with 3 μM pilsicainide for 48 h delayed degradation of WT-hERG proteins and increased the channels expressed on the plasma membrane. A cell membrane-impermeant pilsicainide derivative did not influence the expression of WT-hERG, indicating that pilsicainide stabilized the protein inside the cell. Pilsicainide did not influence phosphorylation of Akt (protein kinase B) or expression of heat shock protein families such as HSF-1, hsp70 and hsp90. E4031, a chemical chaperone for hERG, abolished the pilsicainide effect on hERG. Chronic treatment with pilsicainide could also increase the protein expression of trafficking-defective mutant hERG, G601S and R752W.
Conclusions: Pilsicainide penetrates the plasma membrane, stabilizes WT-hERG proteins by acting as a chemical chaperone, and enhances WT-hERG channel currents. This mechanism could also be applicable to modulations of certain mutant-hERG proteins.
Keywords: Chemical chaperone; Pilsicainide; hERG.
Figures




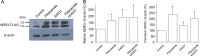

References
-
- Sanguinetti M.C., Jiang C., Curran M.E., Keating M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. - PubMed
-
- Surawicz B. Electrophysiologic substrate of torsade de pointes: dispersion of repolarization or early afterdepolarizations? J Am Coll Cardiol. 1989;14:172–184. - PubMed
-
- Li P., Ninomiya H., Kurata Y. Reciprocal control of hERG stability by hsp70 and hsc70 with implication for restoration of LQT2 mutant stability. Circ Res. 2011;108:458–468. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

