Hypoxia in Obesity and Diabetes: Potential Therapeutic Effects of Hyperoxia and Nitrate
- PMID: 28607631
- PMCID: PMC5457776
- DOI: 10.1155/2017/5350267
Hypoxia in Obesity and Diabetes: Potential Therapeutic Effects of Hyperoxia and Nitrate
Abstract
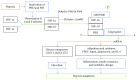
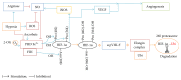
The prevalence of obesity and diabetes is increasing worldwide. Obesity and diabetes are associated with oxidative stress, inflammation, endothelial dysfunction, insulin resistance, and glucose intolerance. Obesity, a chronic hypoxic state that is associated with decreased nitric oxide (NO) bioavailability, is one of the main causes of type 2 diabetes. The hypoxia-inducible factor-1α (HIF-1α) is involved in the regulation of several genes of the metabolic pathways including proinflammatory adipokines, endothelial NO synthase (eNOS), and insulin signaling components. It seems that adipose tissue hypoxia and NO-dependent vascular and cellular dysfunctions are responsible for other consequences linked to obesity-related disorders. Although hyperoxia could reverse hypoxic-related disorders, it increases the production of reactive oxygen species (ROS) and decreases the production of NO. Nitrate can restore NO depletion and has antioxidant properties, and recent data support the beneficial effects of nitrate therapy in obesity and diabetes. Although it seems reasonable to combine hyperoxia and nitrate treatments for managing obesity/diabetes, the combined effects have not been investigated yet. This review discusses some aspects of tissue oxygenation and the potential effects of hyperoxia and nitrate interventions on obesity/diabetes management. It can be proposed that concomitant use of hyperoxia and nitrate is justified for managing obesity and diabetes.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

