A randomized trial of teriflunomide added to glatiramer acetate in relapsing multiple sclerosis
- PMID: 28607708
- PMCID: PMC5433345
- DOI: 10.1177/2055217315618687
A randomized trial of teriflunomide added to glatiramer acetate in relapsing multiple sclerosis
Abstract
Background: Teriflunomide is a once-daily oral immunomodulator for the treatment of relapsing-remitting MS.
Objective: To evaluate the safety and tolerability of teriflunomide as add-on therapy to a stable dose of glatiramer acetate (GA) in patients with relapsing forms of MS (RMS).
Methods: Phase II, randomized, double-blind, add-on, placebo-controlled study. The primary objective was to assess safety and tolerability; secondary objectives were to evaluate effects of treatment on disease activity assessed by MRI and relapse.
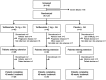
Results: Patients with RMS on GA (N = 123) were randomized 1:1:1 to receive teriflunomide 14 mg (n = 40), 7 mg (n = 42), or placebo (n = 41) for 24 weeks; 96 patients entered the 24-week extension, remaining on original treatment allocation. Teriflunomide was well tolerated over 48 weeks. The frequency of adverse events (AEs) was low across all groups; 5 (12.2%), 3 (7.1%), and 2 (5.0%) patients in the 14 mg, 7 mg, and placebo groups, respectively, discontinued treatment due to AEs. Teriflunomide reduced the number of T1-Gd lesions vs placebo (14 mg: 46.6% relative reduction, p = 0.1931; 7 mg: 64.0%: relative reduction, p = 0.0306).
Conclusions: Teriflunomide added to stable-dose GA had acceptable safety and tolerability, and reduced some MRI markers of disease activity compared with GA alone. NCT00475865 (core study); NCT00811395 (extension).
Keywords: MRI; Randomized clinical trial; multiple sclerosis; relapsing − remitting multiple sclerosis; safety.
Figures

References
-
- sanofi-aventis. Aubagio® (teriflunomide 14 mg tablets). Summary of product characteristics, 2013.
-
- Genzyme Corporation, a Sanofi company. Aubagio® (teriflunomide). Prescribing Information, 2012.
-
- O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293–1303. - PubMed
-
- Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(3): 247–256. - PubMed
-
- Wolinsky JS, Narayana PA, Nelson F, et al. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler 2013; 19: 1310–1319. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

