Cognitive evolution in natalizumab-treated multiple sclerosis patients
- PMID: 28607732
- PMCID: PMC5408753
- DOI: 10.1177/2055217316657116
Cognitive evolution in natalizumab-treated multiple sclerosis patients
Abstract
Background: Cognitive dysfunction affects up to 65% of multiple sclerosis (MS) patients and progresses over time. Natalizumab has been shown to be superior to placebo in preserving cognition for the first two years of therapy.
Objectives: The objectives of this study are to understand the impact of natalizumab on cognition beyond two years of therapy and to investigate whether baseline characteristics are predictive of clinical response.
Methods: This is a single-center, 24-month, observational study. Sixty-three patients treated with natalizumab were assessed prior to monthly infusions using a Cogstate battery and the Symbol Digit Modalities Test (SDMT). Patient demographics were collected at baseline. A linear mixed model was conducted with duration of natalizumab therapy as a between-subjects factor (≤2 or >2 years), assessment as a within-subjects factor, and Multiple Sclerosis Severity Score (MSSS) as a covariate.
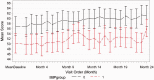
Results: Aside from the MSSS (p = 0.0074), the two groups were identical. No patient showed evidence of sustained cognitive deterioration over the 24-month period. Baseline parameters including impaired cognition did not influence the trajectory of cognitive change over 24 months.
Conclusions: Our results suggest that natalizumab preserves cognition following four to seven years of continuous therapy. This occurs irrespective of baseline characteristics, including impaired cognition.
Keywords: Cognition; multiple sclerosis; natalizumab; relapsing–remitting.
Figures



References
-
- Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991; 41: 685–691. - PubMed
-
- Kujala P, Portin R, Ruutiainen J. The progress of cognitive decline in multiple sclerosis: A controlled 3-year follow-up. Brain 1997; 120(Pt 2): 289–297. - PubMed
-
- Siepman TA, Janssens AC, de Koning I, et al. The role of disability and depression in cognitive functioning within 2 years after multiple sclerosis diagnosis. J Neurol 2008; 255: 910–916. - PubMed
-
- Patti F. Treatment of cognitive impairment in patients with multiple sclerosis. Expert Opin Investig Drugs 2012; 21: 1679–1699. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

