Prevention of Surgical Site Infections and Biofilms: Pharmacokinetics of Subcutaneous Cefazolin and Metronidazole in a Tumescent Lidocaine Solution
- PMID: 28607871
- PMCID: PMC5459654
- DOI: 10.1097/GOX.0000000000001351
Prevention of Surgical Site Infections and Biofilms: Pharmacokinetics of Subcutaneous Cefazolin and Metronidazole in a Tumescent Lidocaine Solution
Abstract
Background: Tumescent anesthesia antibiotic delivery (TAAD) consists of subcutaneous infiltration of antibiotic(s) dissolved tumescent lidocaine anesthesia. Tumescent lidocaine anesthesia contains lidocaine (≤ 1 g/L), epinephrine (≤ 1 mg/L), sodium bicarbonate (10 mEq/L) in 0.9% saline. Our aim was to measure cefazolin and metronidazole concentrations over time in subcutaneous tumescent interstitial fluid (TISF) after TAAD, in serum after TAAD and after intravenous antibiotic delivery (IVAD). We hypothesize that the pharmacokinetic/pharmacodynamic profiles of TAAD + IVAD are superior to IVAD alone for the prevention of surgical site infections and biofilms.
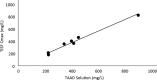
Methods: Concentrations of cefazolin and metronidazole in TISF and serum following TAAD and in serum following IVAD were compared in 5 female volunteers. Subjects received cefazolin or cefazolin plus metronidazole by IVAD alone and by TAAD alone. One subject also received concomitant IVAD and TAAD of these 2 antibiotics. Sequential samples of serum or subcutaneous TISF were assayed for antibiotic concentration.
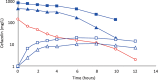
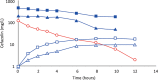
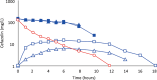
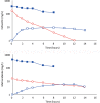
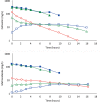
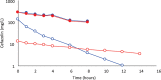
Results: Cefazolin (1 g) by TAAD resulted in an area under the curve of the concentration-time profile and a maximum concentration (Cmax) in subcutaneous tissue that were 16.5 and 5.6 times greater than in serum following 1 g by IVAD. Metronidazole (500 mg) by TAAD resulted in an area under the curve and Cmax that were 8.1 and 24.7 times greater in TISF, than in serum after 500 mg by intravenous delivery. IVAD + TAAD resulted in superior antibiotic concentrations to IVAD alone.
Conclusions: TAAD + IVAD produced superior antibiotic bioavailability in both subcutaneous interstitial fluid and serum compared with IVAD alone. There was no evidence that TAAD of cefazolin and metronidazole poses a significant risk of harm to patients.
Figures



References
-
- Boehm O, Baumgarten G, Hoeft A. Epidemiology of the high-risk population: perioperative risk and mortality after surgery. Curr Opin Crit Care. 2015;21:322–327.. - PubMed
-
- Viet Hung N, Anh Thu T, Rosenthal VD, et al. Surgical site infection rates in seven cities in Vietnam: findings of the International Nosocomial Infection Control Consortium. Surg Infect (Larchmt). 2016;17:243–249.. - PubMed
-
- Toma O, Suntrup P, Stefanescu A, et al. Pharmacokinetics and tissue penetration of cefoxitin in obesity: implications for risk of surgical site infection. Anesth Analg. 2011;113:730–737.. - PubMed
-
- Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659–1669.. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
