Phase I trial of antigen-targeted autologous dendritic cell-based vaccine with in vivo activation of inducible CD40 for advanced prostate cancer
- PMID: 28608115
- PMCID: PMC11029714
- DOI: 10.1007/s00262-017-2027-6
Phase I trial of antigen-targeted autologous dendritic cell-based vaccine with in vivo activation of inducible CD40 for advanced prostate cancer
Abstract
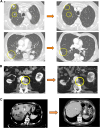
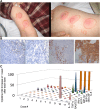
This phase I trial reports the safety and activity of BPX101, a second-generation antigen-targeted autologous antigen presenting cell (APC) vaccine in men with metastatic castration-resistant prostate cancer (mCRPC). To manufacture BPX101, APCs collected in a single leukapheresis were transduced with adenoviral vector Ad5f35 encoding inducible human (ih)-CD40, followed by incubation with protein PA001, which contains the extracellular domain of human prostate-specific membrane antigen. The ih-CD40 represents a modified chimeric version of the dendritic cell (DC) co-stimulatory molecule, CD40, which responds to a bioinert membrane-permeable activating dimerizer drug, rimiducid (AP1903), permitting temporally controlled, lymphoid-localized, DC-specific activation. Eighteen men with progressive mCRPC following ≤1 prior chemotherapy regimen were enrolled to evaluate three doses of BPX101 (4 × 106, 12.5 × 106 and 25 × 106 cells) administered intradermally every 2-4 weeks followed by rimiducid (0.4 mg/kg) intravenous (IV) infusion 24 h after each BPX101 dose. There were no dose-limiting toxicities. Immune upregulation as well as anti-tumor activity was observed with PSA declines, objective tumor regressions and robust efficacy of post-trial therapy. This novel antigen-targeted and in vivo activated immunotherapy platform may warrant further development as monotherapy and as a component of rational combinations.
Keywords: Castration-resistant prostate cancer; Dendritic cell vaccine; Immune response; Metastatic; PSMA; Rimiducid.
Conflict of interest statement
G. Sonpavde: Consultant for Bayer, Sanofi, Pfizer, Novartis, Eisai, Janssen, Amgen, Astrazeneca, Merck, Genentech, Argos, Agensys; research support to institution from Bellicum, Bayer, Onyx, Celgene, Boehringer-Ingelheim, Merck, Pfizer; author for Uptodate; speaker for Clinical Care Options. J.D. McMannis, Y. Bai, M. Seethammagari, J.M.C. Bull, V. Hawkins, T. Dancsak, N. Lapteva, J.M. Levitt: funding to institution by Bellicum Pharmaceuticals, Inc. D.M. Spencer, A. Moseley, K.M. Slawin: employed by and shareholders of Bellicum Pharmaceuticals, Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

