Should we rely on trials with disease- rather than patient-oriented endpoints?
- PMID: 28608246
- PMCID: PMC5924659
- DOI: 10.1007/s00134-017-4859-0
Should we rely on trials with disease- rather than patient-oriented endpoints?
Conflict of interest statement
The authors declare that they have no conflicts of interest.
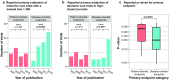
Figures
References
-
- Bucher HC, Guyatt GH, Cook DJ, Evidence-Based Medicine Working Group et al. Users’ guides to the medical literature: XIX. Applying clinical trial results A. How to use an article measuring the effect of an intervention on surrogate end points. JAMA. 1999;282:771–778. doi: 10.1001/jama.282.8.771. - DOI - PubMed
-
- Kellum JA, Zarbock A, Nadim MK. What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med. 2017 - PubMed
-
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (1998) ICH harmonized tripartite guideline: statistical principles for clinical trials E9
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

