The small heat shock protein B8 (HSPB8) efficiently removes aggregating species of dipeptides produced in C9ORF72-related neurodegenerative diseases
- PMID: 28608264
- PMCID: PMC5741577
- DOI: 10.1007/s12192-017-0806-9
The small heat shock protein B8 (HSPB8) efficiently removes aggregating species of dipeptides produced in C9ORF72-related neurodegenerative diseases
Abstract
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are two neurodegenerative diseases in which similar pathogenic mechanisms are involved. Both diseases associate to the high propensity of specific misfolded proteins, like TDP-43 or FUS, to mislocalize and aggregate. This is partly due to their intrinsic biophysical properties and partly as a consequence of failure of the neuronal protein quality control (PQC) system. Several familial ALS/FTD cases are linked to an expansion of a repeated G4C2 hexanucleotide sequence present in the C9ORF72 gene. The G4C2, which localizes in an untranslated region of the C9ORF72 transcript, drives an unconventional repeat-associated ATG-independent translation. This leads to the synthesis of five different dipeptide repeat proteins (DPRs), which are not "classical" misfolded proteins, but generate aberrant aggregation-prone unfolded conformations poorly removed by the PQC system. The DPRs accumulate into p62/SQSTM1 and ubiquitin positive inclusions. Here, we analyzed the biochemical behavior of the five DPRs in immortalized motoneurons. Our data suggest that while the DPRs are mainly processed via autophagy, this system is unable to fully clear their aggregated forms, and thus they tend to accumulate in basal conditions. Overexpression of the small heat shock protein B8 (HSPB8), which facilitates the autophagy-mediated disposal of a large variety of classical misfolded aggregation-prone proteins, significantly decreased the accumulation of most DPR insoluble species. Thus, the induction of HSPB8 might represent a valid approach to decrease DPR-mediated toxicity and maintain motoneuron viability.
Keywords: HSPB8; Motor neuron diseases; Protein aggregation; Protein clearance; RAN translation.
Figures
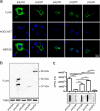

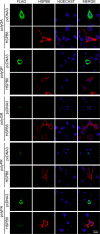
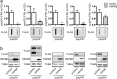
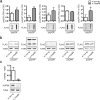
References
-
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, et al. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS Acta. Neuropathology. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. - DOI - PubMed
-
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GGP14039/Fondazione Telethon/International
- 2014-0686/Fondazione Cariplo/International
- ALS_HSPB8/Fondazione Arisla/International
- ALS_Granulopathy/Fondazione Arisla/International
- ALS_Granulopathy/Fondazione AriSLA/International
- ALS_HSPB8/Fondazione AriSLA/International
- 16406/Association Française contre les Myopathies/International
- piano di sviluppo UNIMI - linea B/Università degli studi di Milano/International
- GR-2011-02347198/Ministero della Salute/International
- GR-2011-02347198/Ministero della Salute/International
- Cure_ALS n. 643417/Joint Programme on Neurodegenerative Diseases/International
- CureALS n. 643417/Joint Programme Neurodegenerative Disease/International
- TRANS_ALS/Fondazione regionale per la ricerca biomedica (FRRB), Regione Lombardia/International
- PRIN n. 2015LFPNMN/Ministero dell'Istruzione, dell'Università e della Ricerca/International
- PRIN n. 2015LFPNMN/Ministero dell'Istruzione, dell'Università e della Ricerca/International
- 537 - 2015/European Molecular Biology Organization/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

