A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD
- PMID: 28608438
- PMCID: PMC5659064
- DOI: 10.1002/jcsm.12219
A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD
Abstract
Background: Evidence regarding the efficacy of nutritional supplementation to enhance exercise training responses in COPD patients with low muscle mass is limited. The objective was to study if nutritional supplementation targeting muscle derangements enhances outcome of exercise training in COPD patients with low muscle mass.
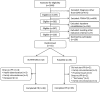
Methods: Eighty-one COPD patients with low muscle mass, admitted to out-patient pulmonary rehabilitation, randomly received oral nutritional supplementation, enriched with leucine, vitamin D, and omega-3 fatty acids (NUTRITION) or PLACEBO as adjunct to 4 months supervised high intensity exercise training.
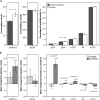
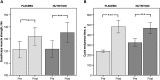
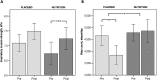
Results: The study population (51% males, aged 43-80) showed moderate airflow limitation, low diffusion capacity, normal protein intake, low plasma vitamin D, and docosahexaenoic acid. Intention-to-treat analysis revealed significant differences after 4 months favouring NUTRITION for body mass (mean difference ± SEM) (+1.5 ± 0.6 kg, P = 0.01), plasma vitamin D (+24%, P = 0.004), eicosapentaenoic acid (+91%,P < 0.001), docosahexaenoic acid (+31%, P < 0.001), and steps/day (+24%, P = 0.048). After 4 months, both groups improved skeletal muscle mass (+0.4 ± 0.1 kg, P < 0.001), quadriceps muscle strength (+12.3 ± 2.3 Nm,P < 0.001), and cycle endurance time (+191.4 ± 34.3 s, P < 0.001). Inspiratory muscle strength only improved in NUTRITION (+0.5 ± 0.1 kPa, P = 0.001) and steps/day declined in PLACEBO (-18%,P = 0.005).
Conclusions: High intensity exercise training is effective in improving lower limb muscle strength and exercise performance in COPD patients with low muscle mass and moderate airflow obstruction. Specific nutritional supplementation had additional effects on nutritional status, inspiratory muscle strength, and physical activity compared with placebo.
Keywords: Emphysema; Muscle function; Nutrient supplementation; Physical activity; Pulmonary rehabilitation.
© 2017 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures
References
-
- Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014. - PubMed
-
- van de Bool C, Gosker HR, van den Borst B, Op den Kamp CM, Slot IG, Schols AM. Muscle quality is more impaired in sarcopenic patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc 2016;17:415–420. - PubMed
-
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–e64. - PubMed
-
- van de Bool C, Steiner MC, Schols AM. Nutritional targets to enhance exercise performance in chronic obstructive pulmonary disease. Curr Opin Clin Nutr Metab Care 2012;15:553–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

