Visible-Light-Mediated [4+2] Annulation of N-Cyclobutylanilines with Alkynes Catalyzed by Self-Doped Ti3+ @TiO2
- PMID: 28608493
- PMCID: PMC5813488
- DOI: 10.1002/chem.201701587
Visible-Light-Mediated [4+2] Annulation of N-Cyclobutylanilines with Alkynes Catalyzed by Self-Doped Ti3+ @TiO2
Abstract
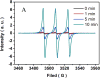
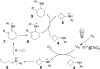
We herein report a visible-light-mediated heterogeneous [4+2] annulation of N-cyclobutylanilines with alkynes catalyzed by self-doped Ti3+ @TiO2 . The self-doped Ti3+ @TiO2 is stable under photooxidation conditions, easy to recycle, and can be used multiple times without appreciable loss of activity. Extensive mechanistic studies suggest that the annulation reaction is mediated by singlet oxygen, which is generated through the photosensitization of oxygen in the air by the self-doped Ti3+ @TiO2 . In contrast, the homogeneous variant catalyzed by a far more expensive iridium complex proceeds under an inert atmosphere, which indicates a different mechanism. The substrate scopes of the two processes are comparable.
Keywords: annulation; cyclobutylaniline; photocatalysis; singlet oxygen; titanium; visible light.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





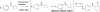
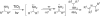


Similar articles
-
The Cleavage of a C-C Bond in Cyclobutylanilines by Visible-Light Photoredox Catalysis: Development of a [4+2] Annulation Method.Angew Chem Int Ed Engl. 2015 Sep 21;54(39):11424-7. doi: 10.1002/anie.201504076. Epub 2015 Jul 24. Angew Chem Int Ed Engl. 2015. PMID: 26228259 Free PMC article.
-
Facile One-Step Route for the Development of in Situ Cocatalyst-Modified Ti3+ Self-Doped TiO2 for Improved Visible-Light Photocatalytic Activity.ACS Appl Mater Interfaces. 2016 Oct 19;8(41):27642-27653. doi: 10.1021/acsami.6b07000. Epub 2016 Oct 5. ACS Appl Mater Interfaces. 2016. PMID: 27667775
-
Carbon coating stabilized Ti(3+)-doped TiO2 for photocatalytic hydrogen generation under visible light irradiation.Dalton Trans. 2015 Jul 28;44(28):12812-7. doi: 10.1039/c5dt01204j. Epub 2015 Jun 22. Dalton Trans. 2015. PMID: 26098384
-
Visible light-induced photocatalytic and antibacterial activity of N-doped TiO2.J Biomed Mater Res B Appl Biomater. 2020 Feb;108(2):451-459. doi: 10.1002/jbm.b.34401. Epub 2019 May 14. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31087638
-
Embracing heterogeneous photocatalysis: evolution of photocatalysts in annulation of dimethylanilines and maleimides.Chem Commun (Camb). 2024 Aug 13;60(66):8645-8657. doi: 10.1039/d4cc02516d. Chem Commun (Camb). 2024. PMID: 39078307 Review.
Cited by
-
Visible-light-enabled stereoselective synthesis of functionalized cyclohexylamine derivatives via [4 + 2] cycloadditions.Chem Sci. 2024 Mar 26;15(17):6507-6514. doi: 10.1039/d4sc00667d. eCollection 2024 May 1. Chem Sci. 2024. PMID: 38699278 Free PMC article.
-
Photochemical Formal (4 + 2)-Cycloaddition of Imine-Substituted Bicyclo[1.1.1]pentanes and Alkenes.J Am Chem Soc. 2021 Dec 22;143(50):21223-21228. doi: 10.1021/jacs.1c10541. Epub 2021 Dec 13. J Am Chem Soc. 2021. PMID: 34902245 Free PMC article.
References
-
- Akira F, Kenichi H. Bull. Chem. Chem. Soc. Jpn. 1971;44:1148–1150.
- Fujishima A, Honda K. Nature. 1972;238:37–38. - PubMed
-
- Schneider J, Bahnemann D, Ye J, Puma GL, Dionysiou in DD. RSC Energy and Environment Series. Vol. 14. Royal Society of Chemistry; 2016.
- Dionysiou DD, Puma GL, Ye J, Schneider J, Bahnemann D. RSC Energy and Environment Series. Vol. 15. Royal Society of Chemistry; 2016.
-
- Manley DW, Walton JC. Beilstein. J. Org. Chem. 2015;11:1570–1582. - PMC - PubMed
- Lang X, Ma W, Chen C, Ji H, Zhao J. Acc. Chem. Res. 2014;47:355–363. - PubMed
- Lang X, Chen X, Zhao J. Chem. Soc. Rev. 2014;43:473–486. - PubMed
- Friedmann D, Hakki A, Kim H, Choi W, Bahnemann D. Green Chem. 2016;18:5391–5411.
-
- Kisch H. Angew. Chem. Int. Ed. 2013;52:812–847. Angew. Chem.2013, 125, 842–879. - PubMed
-
-
For selected recent reviews on doping of TiO2: Anpo M, Takeuchi M. J. Catal. 2003;216:505–516.Dahl M, Liu Y, Yin Y. Chem. Rev. 2014;114:9853–9889.Asahi R, Morikawa T, Irie H, Ohwaki T. Chem. Rev. 2014;114:9824–9852.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

