Endocannabinoid system in neurodegenerative disorders
- PMID: 28608560
- PMCID: PMC5669051
- DOI: 10.1111/jnc.14098
Endocannabinoid system in neurodegenerative disorders
Abstract
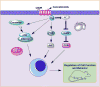
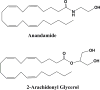
Most neurodegenerative disorders (NDDs) are characterized by cognitive impairment and other neurological defects. The definite cause of and pathways underlying the progression of these NDDs are not well-defined. Several mechanisms have been proposed to contribute to the development of NDDs. These mechanisms may proceed concurrently or successively, and they differ among cell types at different developmental stages in distinct brain regions. The endocannabinoid system, which involves cannabinoid receptors type 1 (CB1R) and type 2 (CB2R), endogenous cannabinoids and the enzymes that catabolize these compounds, has been shown to contribute to the development of NDDs in several animal models and human studies. In this review, we discuss the functions of the endocannabinoid system in NDDs and converse the therapeutic efficacy of targeting the endocannabinoid system to rescue NDDs.
Keywords: Alzheimer's disease; CB1 receptors; Huntington's disease; Loss of neurons; Parkinson's disease; motor and memory behavior.
© 2017 International Society for Neurochemistry.
Conflict of interest statement
Conflicts of interest: None
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> otherwise insert info unless it is already included
The authors declare no competing financial interests. All experiments were conducted in compliance with the ARRIVE guidelines.
Figures


References
-
- Ahmad R, Goffin K, Van den Stock J, et al. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur Neuropsychopharmacol. 2014;24:242–250. - PubMed
-
- Ahmad R, Postnov A, Bormans G, Versijpt J, Vandenbulcke M, Van Laere K. Decreased in vivo availability of the cannabinoid type 2 receptor in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2016;43:2219–2227. - PubMed
-
- Allen KL, Waldvogel HJ, Glass M, Faull RL. Cannabinoid (CB(1)), GABA(A) and GABA(B) receptor subunit changes in the globus pallidus in Huntington’s disease. J Chem Neuroanat. 2009;37:266–281. - PubMed
-
- Aso E, Andres-Benito P, Carmona M, Maldonado R, Ferrer I. Cannabinoid Receptor 2 Participates in Amyloid-beta Processing in a Mouse Model of Alzheimer’s Disease but Plays a Minor Role in the Therapeutic Properties of a Cannabis-Based Medicine. Journal of Alzheimer’s disease: JAD. 2016;51:489–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

