Enhanced Osteogenic Differentiation in Zoledronate-Treated Osteoporotic Patients
- PMID: 28608802
- PMCID: PMC5486083
- DOI: 10.3390/ijms18061261
Enhanced Osteogenic Differentiation in Zoledronate-Treated Osteoporotic Patients
Abstract
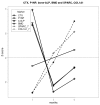
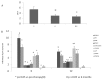
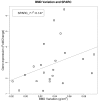
Bisphosphonates are well known inhibitors of osteoclast activity and thus may be employed to influence osteoblast activity. The present study was designed to evaluate the in vivo effects of zoledronic acid (ZA) on the proliferation and osteoblastic commitment of mesenchymal stem cells (MSC) in osteoporotic patients. We studied 22 postmenopausal osteoporotic patients. Densitometric, biochemical, cellular and molecular data were collected before as well as after 6 and 12 months of ZA treatment. Peripheral blood MSC-like cells were quantified by colony-forming unit fibroblastic assay; their osteogenic differentiation potential was evaluated after 3 and 7 days of induction, respectively. Circulating MSCs showed significantly increased expression levels of osteoblastic marker genes such as Runt-related transcription factor 2 (RUNX2), and Osteonectin (SPARC) during the 12 months of monitoring time. Lumbar bone mineral density (BMD) variation and SPARC gene expression correlated positively. Bone turnover marker levels were significantly lowered after ZA treatment; the effect was more pronounced for C terminal telopeptide (CTX) than for Procollagen Type 1 N-Terminal Propeptide (P1NP) and bone alkaline phosphatase (bALP). Our findings suggest a discrete anabolic activity supported by osteogenic commitment of MSCs, consequent to ZA treatment. We confirm its anabolic effects in vivo on osteogenic precursors.
Keywords: Runt-related transcription factor 2 (RUNX2); bone turnover; differentiation; gene expression; mesenchymal stem cells; zoledronic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Richards J.B., Leslie W.D., Joseph L., Siminoski K., Hanley D.A., Adachi J.D., Brown J.P., Morin S., Papaioannou A., Josse R.G., et al. Changes to osteoporosis prevalence according to method of risk assessment. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007;22:228–234. doi: 10.1359/jbmr.061109. - DOI - PubMed
-
- Lecanda F., Avioli L.V., Cheng S.L. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J. Cell. Biochem. 1997;67:386–396. doi: 10.1002/(SICI)1097-4644(19971201)67:3<386::AID-JCB10>3.0.CO;2-B. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

