The Unique Role of the ECERIFERUM2-LIKE Clade of the BAHD Acyltransferase Superfamily in Cuticular Wax Metabolism
- PMID: 28608803
- PMCID: PMC5489795
- DOI: 10.3390/plants6020023
The Unique Role of the ECERIFERUM2-LIKE Clade of the BAHD Acyltransferase Superfamily in Cuticular Wax Metabolism
Abstract
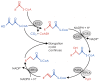
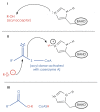
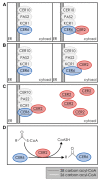
The elongation of very-long-chain fatty acids is a conserved process used for the production of many metabolites, including plant cuticular waxes. The elongation of precursors of the most abundant cuticular wax components of some plants, however, is unique in requiring ECERIFERUM2-LIKE (CER2-LIKE) proteins. CER2-LIKEs are a clade within the BAHD superfamily of acyltransferases. They are known to be required for cuticular wax production in both Arabidopsis and maize based on mutant studies. Heterologous expression of Arabidopsis and rice CER2-LIKEs in Saccharomyces cerevisiae has demonstrated that they modify the chain-length specificity of elongation when paired with particular condensing enzymes. Despite sequence homology, CER2-LIKEs are distinct from the BAHD superfamily in that they do not appear to use acyl transfer activity to fulfill their biological function. Here, we review the discovery and characterization of CER2-LIKEs, propose several models to explain their function, and explore the importance of CER2-LIKE proteins for the evolution of plant cuticles.
Keywords: BAHD acyltransferase; cuticle; eceriferum; elongation; evolution; very-long-chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bach L., Michaelson L.V., Haslam R., Bellec Y., Gissot L., Marion J., Da Costa M., Boutin J.P., Miquel M., Tellier F., et al. The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proc. Natl. Acad. Sci. USA. 2008;105:14727–14731. doi: 10.1073/pnas.0805089105. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

