Predicting Outcome and Therapy Response in mCRC Patients Using an Indirect Method for CTCs Detection by a Multigene Expression Panel: A Multicentric Prospective Validation Study
- PMID: 28608814
- PMCID: PMC5486087
- DOI: 10.3390/ijms18061265
Predicting Outcome and Therapy Response in mCRC Patients Using an Indirect Method for CTCs Detection by a Multigene Expression Panel: A Multicentric Prospective Validation Study
Abstract
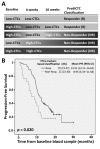
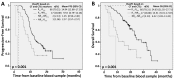
Colorectal cancer (CRC) is one of the major causes of cancer-related deaths. Early detection of tumor relapse is crucial for determining the most appropriate therapeutic management. In clinical practice, computed tomography (CT) is routinely used, but small tumor changes are difficult to visualize, and reliable blood-based prognostic and monitoring biomarkers are urgently needed. The aim of this study was to prospectively validate a gene expression panel (composed of GAPDH, VIL1, CLU, TIMP1, TLN1, LOXL3 and ZEB2) for detecting circulating tumor cells (CTCs) as prognostic and predictive tool in blood samples from 94 metastatic CRC (mCRC) patients. Patients with higher gene panel expression before treatment had a reduced progression-free survival (PFS) and overall-survival (OS) rates compared with patients with low expression (p = 0.003 and p ≤ 0.001, respectively). Patients with increased expression of CTCs markers during treatment presented PFS and OS times of 8.95 and 11.74 months, respectively, compared with 14.41 and 24.7 for patients presenting decreased expression (PFS; p = 0.020; OS; p ≤ 0.001). Patients classified as non-responders by CTCs with treatment, but classified as responders by CT scan, showed significantly shorter survival times (PFS: 8.53 vs. 11.70; OS: 10.37 vs. 24.13; months). In conclusion, our CTCs detection panel demonstrated efficacy for early treatment response assessment in mCRC patients, and with increased reliability compared to CT scan.
Keywords: biomarkers; circulating tumor cells; metastatic colorectal cancer; therapy response.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer.Int J Cancer. 2014 Dec 1;135(11):2633-43. doi: 10.1002/ijc.28910. Epub 2014 Apr 29. Int J Cancer. 2014. PMID: 24752533
-
Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer.J Clin Oncol. 2008 Jul 1;26(19):3213-21. doi: 10.1200/JCO.2007.15.8923. J Clin Oncol. 2008. PMID: 18591556
-
Improved Detection of Circulating Tumor Cells in Metastatic Colorectal Cancer by the Combination of the CellSearch® System and the AdnaTest®.PLoS One. 2016 May 16;11(5):e0155126. doi: 10.1371/journal.pone.0155126. eCollection 2016. PLoS One. 2016. PMID: 27182774 Free PMC article.
-
Systemic Therapy for Metastatic Colorectal Cancer: From Current Standards to Future Molecular Targeted Approaches.Am Soc Clin Oncol Educ Book. 2017;37:246-256. doi: 10.1200/EDBK_175679. Am Soc Clin Oncol Educ Book. 2017. PMID: 28561718 Review.
-
Circulating Tumor Cells in Colorectal Cancer: Detection Systems and Clinical Utility.Int J Mol Sci. 2022 Nov 5;23(21):13582. doi: 10.3390/ijms232113582. Int J Mol Sci. 2022. PMID: 36362369 Free PMC article. Review.
Cited by
-
Low expression of Talin1 is associated with advanced pathological features in colorectal cancer patients.Sci Rep. 2020 Oct 20;10(1):17786. doi: 10.1038/s41598-020-74810-6. Sci Rep. 2020. PMID: 33082414 Free PMC article.
-
Current Challenges of Methylation-Based Liquid Biopsies in Cancer Diagnostics.Cancers (Basel). 2024 May 24;16(11):2001. doi: 10.3390/cancers16112001. Cancers (Basel). 2024. PMID: 38893121 Free PMC article. Review.
-
Proteins: Neglected active ingredients in edible bird's nest.Chin Herb Med. 2023 May 16;15(3):383-390. doi: 10.1016/j.chmed.2023.02.004. eCollection 2023 Jul. Chin Herb Med. 2023. PMID: 37538855 Free PMC article. Review.
-
Methylation detection of circulating tumor cell miR-486-5p/miR-34c-5p in the progression of colorectal cancer.Clin Transl Oncol. 2023 Mar;25(3):673-684. doi: 10.1007/s12094-022-02973-x. Epub 2022 Oct 15. Clin Transl Oncol. 2023. PMID: 36243896
-
Genome-wide analysis of cell-Free DNA methylation profiling with MeDIP-seq identified potential biomarkers for colorectal cancer.World J Surg Oncol. 2022 Jan 22;20(1):21. doi: 10.1186/s12957-022-02487-4. World J Surg Oncol. 2022. PMID: 35065650 Free PMC article.
References
-
- Sobrero A.F., Maurel J., Fehrenbacher L., Scheithauer W., Abubakr Y.A., Lutz M.P., Vega-Villegas M.E., Eng C., Steinhauer E.U., Prausova J., et al. EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. - DOI - PubMed
-
- Cassidy J., Clarke S., Díaz-Rubio E., Scheithauer W., Figer A., Wong R., Koski S., Lichinitser M., Yang T.-S., Rivera F., et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

