Microgroove and Collagen-poly(ε-caprolactone) Nanofiber Mesh Coating Improves the Mechanical Stability and Osseointegration of Titanium Implants
- PMID: 28608839
- PMCID: PMC5485792
- DOI: 10.3390/nano7060145
Microgroove and Collagen-poly(ε-caprolactone) Nanofiber Mesh Coating Improves the Mechanical Stability and Osseointegration of Titanium Implants
Abstract
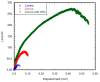
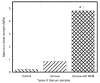
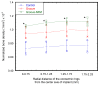
The effect of depositing a collagen (CG)-poly-ε-caprolactone (PCL) nanofiber mesh (NFM) at the microgrooves of titanium (Ti) on the mechanical stability and osseointegration of the implant with bone was investigated using a rabbit model. Three groups of Ti samples were produced: control Ti samples where there were no microgrooves or CG-PCL NFM, groove Ti samples where microgrooves were machined on the circumference of Ti, and groove-NFM Ti samples where CG-PCL NFM was deposited on the machined microgrooves. Each group of Ti samples was implanted in the rabbit femurs for eight weeks. The mechanical stability of the Ti/bone samples were quantified by shear strength from a pullout tension test. Implant osseointegration was evaluated by a histomorphometric analysis of the percentage of bone and connective tissue contact with the implant surface. The bone density around the Ti was measured by micro-computed tomography (μCT) analysis. This study found that the shear strength of groove-NFM Ti/bone samples was significantly higher compared to control and groove Ti/bone samples (p < 0.05) and NFM coating influenced the bone density around Ti samples. In vivo histomorphometric analyses show that bone growth into the Ti surface increased by filling the microgrooves with CG-PCL NFM. The study concludes that a microgroove assisted CG-PCL NFM coating may benefit orthopedic implants.
Keywords: bone; electrospun nanofiber; in vivo study; shear strength; titanium.
Conflict of interest statement
The authors have no conflict of interest.
Figures








Similar articles
-
Effect of Collagen-Polycaprolactone Nanofibers Matrix Coating on the In Vitro Cytocompatibility and In Vivo Bone Responses of Titanium.J Med Biol Eng. 2018 Apr;38(2):197-210. doi: 10.1007/s40846-017-0312-7. Epub 2017 Jul 24. J Med Biol Eng. 2018. PMID: 29861706 Free PMC article.
-
Osseointegration and mechanical stability of pyrocarbon and titanium hand implants in a load-bearing in vivo model for small joint arthroplasty.J Hand Surg Am. 2006 Jan;31(1):90-7. doi: 10.1016/j.jhsa.2005.10.002. J Hand Surg Am. 2006. PMID: 16443111
-
Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection.Biomed Mater. 2017 Jul 5;12(4):045008. doi: 10.1088/1748-605X/aa6a26. Biomed Mater. 2017. PMID: 28357996
-
Osseointegration of zirconia and titanium implants in a rabbit tibiae model evaluated by microtomography, histomorphometry and fluorochrome labeling analyses.J Periodontal Res. 2018 Apr;53(2):210-221. doi: 10.1111/jre.12508. Epub 2017 Oct 17. J Periodontal Res. 2018. PMID: 29044523
-
Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved Surfaces and Graphene Oxide Coating.ACS Appl Mater Interfaces. 2019 Oct 30;11(43):39470-39483. doi: 10.1021/acsami.9b12733. Epub 2019 Oct 17. ACS Appl Mater Interfaces. 2019. PMID: 31594306
Cited by
-
Laser-Induced Microgrooves Improve the Mechanical Responses of Cemented Implant Systems.Micromachines (Basel). 2020 Apr 29;11(5):466. doi: 10.3390/mi11050466. Micromachines (Basel). 2020. PMID: 32365464 Free PMC article.
-
Strategies for Biomaterial-Based Spinal Cord Injury Repair via the TLR4-NF-κB Signaling Pathway.Front Bioeng Biotechnol. 2022 Apr 29;9:813169. doi: 10.3389/fbioe.2021.813169. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35600111 Free PMC article. Review.
-
Fatigue behavior of zirconia with microgrooved surfaces produced using femtosecond laser.Lasers Med Sci. 2023 Jan 4;38(1):33. doi: 10.1007/s10103-022-03679-w. Lasers Med Sci. 2023. PMID: 36598586
-
Biological Safety Evaluation and Surface Modification of Biocompatible Ti-15Zr-4Nb Alloy.Materials (Basel). 2021 Feb 4;14(4):731. doi: 10.3390/ma14040731. Materials (Basel). 2021. PMID: 33557312 Free PMC article.
-
[Effects of femtosecond laser treatment on surface characteristics and flexural strength of zirconia].Beijing Da Xue Xue Bao Yi Xue Ban. 2021 Aug 18;53(4):770-775. doi: 10.19723/j.issn.1671-167X.2021.04.025. Beijing Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34393243 Free PMC article. Chinese.
References
-
- Brunette D.M., Tengvall P., Textor M., Thomsen P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. Springer Science & Business Media; Berlin, Germany: 2001.
-
- Im B.J., Lee S.W., Oh N., Lee M.H., Kang J.H., Leesungbok R., Lee S.C., Ahn S.J., Park J.S. Texture direction of combined microgrooves and submicroscale topographies of titanium substrata influence adhesion, proliferation, and differentiation in human primary cells. Arch. Oral Biol. 2012;57:898–905. doi: 10.1016/j.archoralbio.2011.11.013. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

