Cellular senescence drives age-dependent hepatic steatosis
- PMID: 28608850
- PMCID: PMC5474745
- DOI: 10.1038/ncomms15691
Cellular senescence drives age-dependent hepatic steatosis
Abstract
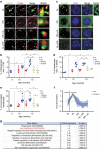
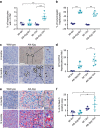
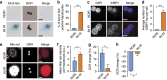
The incidence of non-alcoholic fatty liver disease (NAFLD) increases with age. Cellular senescence refers to a state of irreversible cell-cycle arrest combined with the secretion of proinflammatory cytokines and mitochondrial dysfunction. Senescent cells contribute to age-related tissue degeneration. Here we show that the accumulation of senescent cells promotes hepatic fat accumulation and steatosis. We report a close correlation between hepatic fat accumulation and markers of hepatocyte senescence. The elimination of senescent cells by suicide gene-meditated ablation of p16Ink4a-expressing senescent cells in INK-ATTAC mice or by treatment with a combination of the senolytic drugs dasatinib and quercetin (D+Q) reduces overall hepatic steatosis. Conversely, inducing hepatocyte senescence promotes fat accumulation in vitro and in vivo. Mechanistically, we show that mitochondria in senescent cells lose the ability to metabolize fatty acids efficiently. Our study demonstrates that cellular senescence drives hepatic steatosis and elimination of senescent cells may be a novel therapeutic strategy to reduce steatosis.
Conflict of interest statement
Patents on INK-ATTAC mice and senolytic drugs are held by Mayo Clinic and licensed to Unity Biotechnology. J.L.K., A.P. and T.T. may gain financially from these patents and licenses. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic conflict of interest policies. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Senescence explains age- and obesity-related liver steatosis.Cell Stress. 2017 Sep 19;1(1):70-72. doi: 10.15698/cst2017.10.108. Cell Stress. 2017. PMID: 31225436 Free PMC article.
References
-
- Hardy T., Oakley F., Anstee Q. M. & Day C. P. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu. Rev. Pathol. 11, 451–496 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

