Effect of Low-Dose Ferrous Sulfate vs Iron Polysaccharide Complex on Hemoglobin Concentration in Young Children With Nutritional Iron-Deficiency Anemia: A Randomized Clinical Trial
- PMID: 28609534
- PMCID: PMC5815003
- DOI: 10.1001/jama.2017.6846
Effect of Low-Dose Ferrous Sulfate vs Iron Polysaccharide Complex on Hemoglobin Concentration in Young Children With Nutritional Iron-Deficiency Anemia: A Randomized Clinical Trial
Abstract
Importance: Iron-deficiency anemia (IDA) affects millions of persons worldwide, and is associated with impaired neurodevelopment in infants and children. Ferrous sulfate is the most commonly prescribed oral iron despite iron polysaccharide complex possibly being better tolerated.
Objective: To compare the effect of ferrous sulfate with iron polysaccharide complex on hemoglobin concentration in infants and children with nutritional IDA.
Design, setting, and participants: Double-blind, superiority randomized clinical trial of infants and children aged 9 to 48 months with nutritional IDA (assessed by history and laboratory criteria) that was conducted in an outpatient hematology clinic at a US tertiary care hospital from September 2013 through November 2015; 12-week follow-up ended in January 2016.
Interventions: Three mg/kg of elemental iron once daily as either ferrous sulfate drops or iron polysaccharide complex drops for 12 weeks.
Main outcomes and measures: Primary outcome was change in hemoglobin over 12 weeks. Secondary outcomes included complete resolution of IDA (defined as hemoglobin concentration >11 g/dL, mean corpuscular volume >70 fL, reticulocyte hemoglobin equivalent >25 pg, serum ferritin level >15 ng/mL, and total iron-binding capacity <425 μg/dL at the 12-week visit), changes in serum ferritin level and total iron-binding capacity, adverse effects.
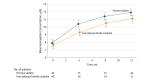
Results: Of 80 randomized infants and children (median age, 22 months; 55% male; 61% Hispanic white; 40 per group), 59 completed the trial (28 [70%] in ferrous sulfate group; 31 [78%] in iron polysaccharide complex group). From baseline to 12 weeks, mean hemoglobin increased from 7.9 to 11.9 g/dL (ferrous sulfate group) vs 7.7 to 11.1 g/dL (iron complex group), a greater difference of 1.0 g/dL (95% CI, 0.4 to 1.6 g/dL; P < .001) with ferrous sulfate (based on a linear mixed model). Proportion with a complete resolution of IDA was higher in the ferrous sulfate group (29% vs 6%; P = .04). Median serum ferritin level increased from 3.0 to 15.6 ng/mL (ferrous sulfate) vs 2.0 to 7.5 ng/mL (iron complex) over 12 weeks, a greater difference of 10.2 ng/mL (95% CI, 6.2 to 14.1 ng/mL; P < .001) with ferrous sulfate. Mean total iron-binding capacity decreased from 501 to 389 μg/dL (ferrous sulfate) vs 506 to 417 μg/dL (iron complex) (a greater difference of -50 μg/dL [95% CI, -86 to -14 μg/dL] with ferrous sulfate; P < .001). There were more reports of diarrhea in the iron complex group than in the ferrous sulfate group (58% vs 35%, respectively; P = .04).
Conclusions and relevance: Among infants and children aged 9 to 48 months with nutritional iron-deficiency anemia, ferrous sulfate compared with iron polysaccharide complex resulted in a greater increase in hemoglobin concentration at 12 weeks. Once daily, low-dose ferrous sulfate should be considered for children with nutritional iron-deficiency anemia.
Trial registration: clinicaltrials.gov Identifier: NCT01904864.
Conflict of interest statement
Figures
Comment in
-
Effect of Different Iron Preparations for Young Children With Iron-Deficiency Anemia.JAMA. 2017 Oct 3;318(13):1282. doi: 10.1001/jama.2017.11933. JAMA. 2017. PMID: 28973242 No abstract available.
References
-
- McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454. - PubMed
-
- Brotanek JM, Gosz J, Weitzman M, Flores G. Secular trends in the prevalence of iron deficiency among US toddlers, 1976-2002. Arch Pediatr Adolesc Med. 2008;162(4):374-381. - PubMed
-
- McDonagh MS, Blazina I, Dana T, et al. Screening and routine supplementation for iron deficiency anemia. Pediatrics. 2015;135(4):723-733. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

