The many faces of Pluripotency: in vitro adaptations of a continuum of in vivo states
- PMID: 28610558
- PMCID: PMC5470286
- DOI: 10.1186/s12861-017-0150-4
The many faces of Pluripotency: in vitro adaptations of a continuum of in vivo states
Abstract
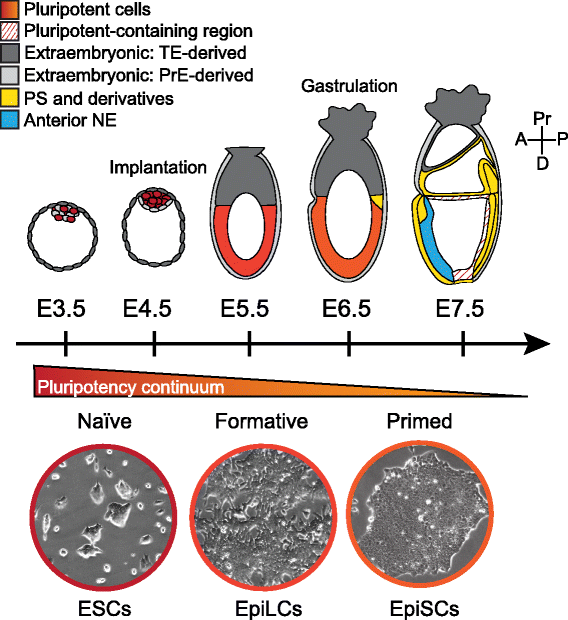
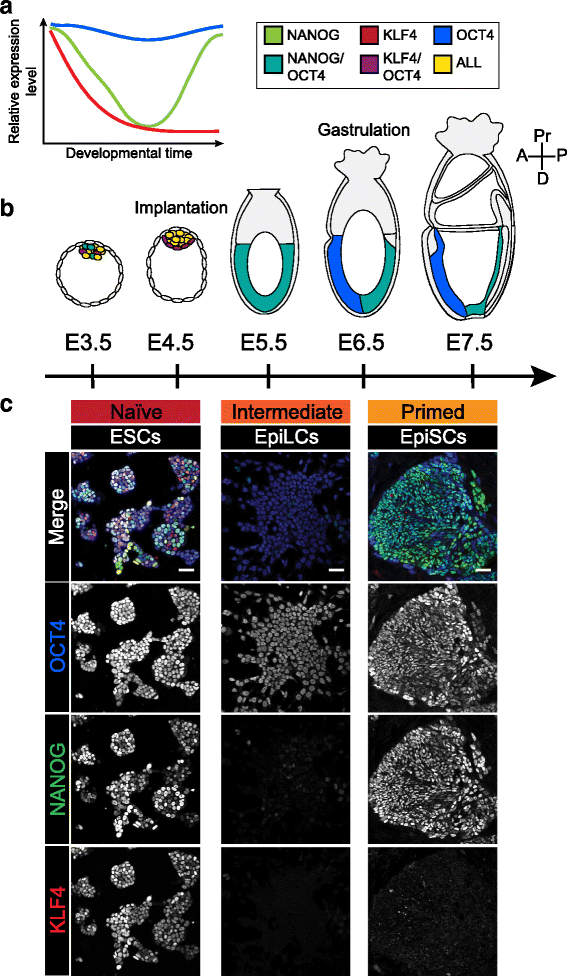
Pluripotency defines the propensity of a cell to differentiate into, and generate, all somatic, as well as germ cells. The epiblast of the early mammalian embryo is the founder population of all germ layer derivatives and thus represents the bona fide in vivo pluripotent cell population. The so-called pluripotent state spans several days of development and is lost during gastrulation as epiblast cells make fate decisions towards a mesoderm, endoderm or ectoderm identity. It is now widely recognized that the features of the pluripotent population evolve as development proceeds from the pre- to post-implantation period, marked by distinct transcriptional and epigenetic signatures. During this period of time epiblast cells mature through a continuum of pluripotent states with unique properties. Aspects of this pluripotent continuum can be captured in vitro in the form of stable pluripotent stem cell types. In this review we discuss the continuum of pluripotency existing within the mammalian embryo, using the mouse as a model, and the cognate stem cell types that can be derived and propagated in vitro. Furthermore, we speculate on embryonic stage-specific characteristics that could be utilized to identify novel, developmentally relevant, pluripotent states.
Keywords: Chimaera; Embryonic stem cells; Epiblast stem cells; Epiblast-like cells; Formative; Ground state; Intermediate; Naïve; Pluripotency; Primed.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

