Blended care vs. usual care in the treatment of depressive symptoms and disorders in general practice [BLENDING]: study protocol of a non-inferiority randomized trial
- PMID: 28610561
- PMCID: PMC5470276
- DOI: 10.1186/s12888-017-1376-1
Blended care vs. usual care in the treatment of depressive symptoms and disorders in general practice [BLENDING]: study protocol of a non-inferiority randomized trial
Abstract
Background: The majority of patients with depressive disorders are treated by general practitioners (GPs) and are prescribed antidepressant medication. Patients prefer psychological treatments but they are under-used, mainly due to time constraints and limited accessibility. A promising approach to deliver psychological treatment is blended care, i.e. guided online treatment. However, the cost-effectiveness of blended care formatted as an online psychological treatment supported by the patients' own GP or general practice mental health worker (MHW) in routine primary care is unknown. We aim to demonstrate non-inferiority of blended care compared with usual care in patients with depressive symptoms or a depressive disorder in general practice. Additionally, we will explore the real-time course over the day of emotions and affect, and events within individuals during treatment.
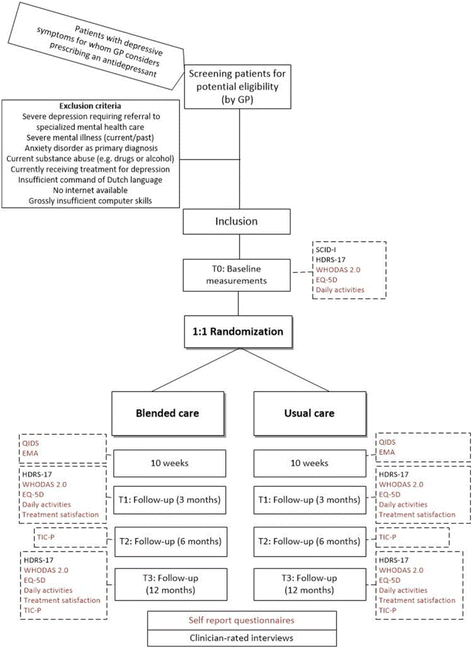
Methods: This is a pragmatic non-inferiority trial including 300 patients with depressive symptoms, recruited by collaborating GPs and MHWs. After inclusion, participants are randomized to either blended care or usual care in routine general practice. Blended care consists of the 'Act and Feel' treatment: an eight-week web-based program based on behavioral activation with integrated monitoring of depressive symptomatology and automatized feedback. GPs or their MHWs coach the participants through regular face-to-face or telephonic consultations with at least three sessions. Depressive symptomatology, health status, functional impairment, treatment satisfaction, daily activities and resource use are assessed during a follow-up period of 12 months. During treatment, real-time fluctuations in emotions and affect, and daily events will be rated using ecological momentary assessment. The primary outcome is the reduction of depressive symptoms from baseline to three months follow-up. We will conduct intention-to-treat analyses and supplementary per-protocol analyses.
Discussion: This trial will show whether blended care might be an appropriate treatment strategy for patients with depressive symptoms and depressive disorder in general practice.
Trial registration: Netherlands Trial Register: NTR4757; 25 August 2014. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4757 . (Archived by WebCite® at http://www.webcitation.org/6mnXNMGef ).
Keywords: Blended care; Cost-effectiveness; Depression; Depressive disorders; Depressive symptoms; Effectiveness; General practice; Internet-based treatment; Primary care; eHealth.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

