Complete human serum maintains viability and chondrogenic potential of human synovial stem cells: suitable conditions for transplantation
- PMID: 28610596
- PMCID: PMC5470274
- DOI: 10.1186/s13287-017-0596-0
Complete human serum maintains viability and chondrogenic potential of human synovial stem cells: suitable conditions for transplantation
Abstract
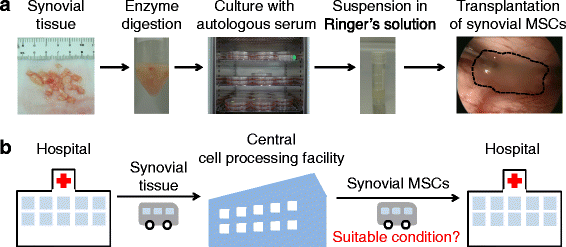
Background: In our clinical practice, we perform transplantations of autologous synovial mesenchymal stem cells (MSCs) for cartilage and meniscus regenerative medicine. One of the most important issues to ensuring clinical efficacy involves the transport of synovial MSCs from the processing facility to the clinic. Complete human serum (100% human serum) is an attractive candidate material in which to suspend synovial MSCs for their preservation during transport. The purpose of this study was to investigate whether complete human serum maintained MSC viability and chondrogenic potential and to examine the optimal temperature conditions for the preservation of human synovial MSCs.
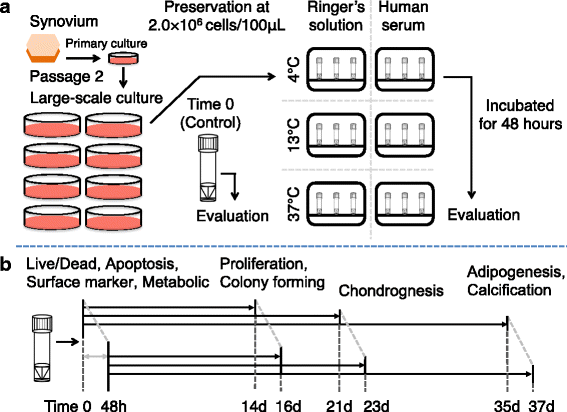
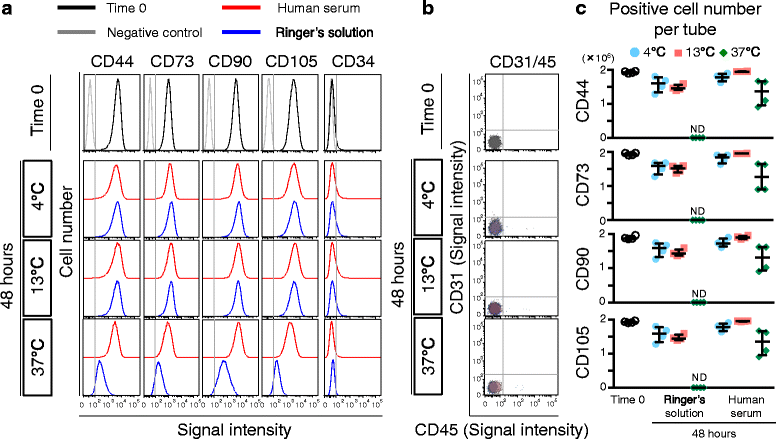
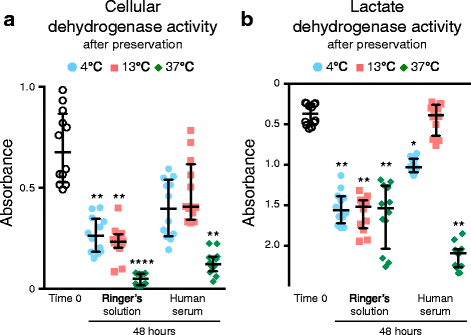
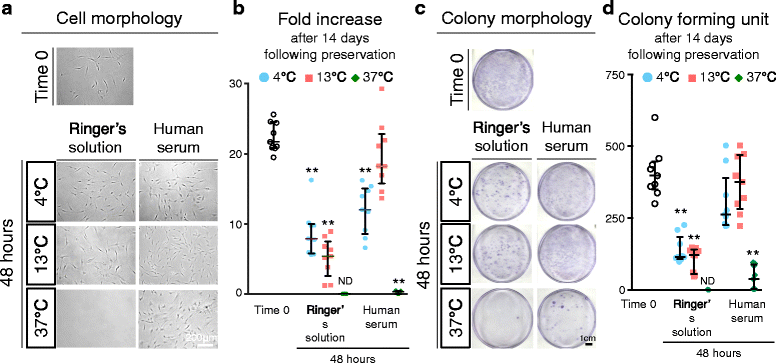
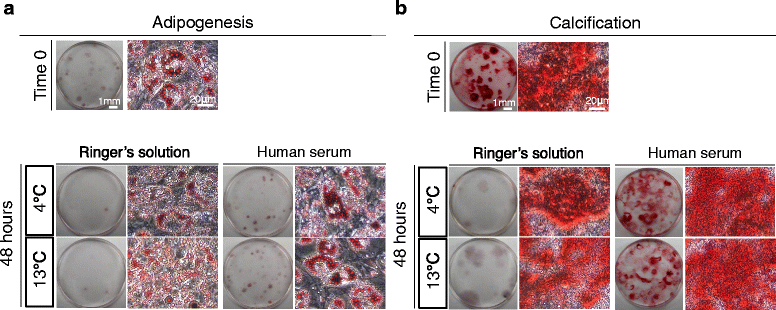
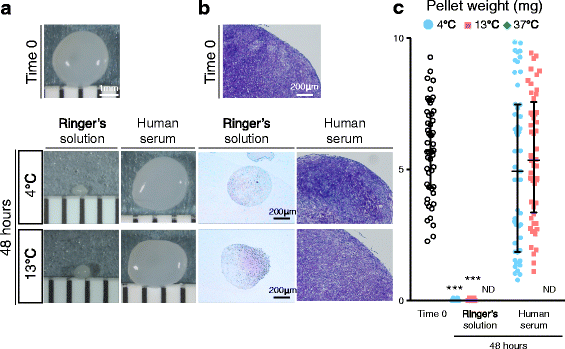
Methods: Human synovium was harvested from the knees of 14 donors with osteoarthritis during total knee arthroplasty. Passage 2 synovial MSCs were suspended at 2 million cells/100 μL in Ringer's solution or complete human serum at 4, 13, and 37 °C for 48 h. These cells were analyzed for live cell rates, cell surface marker expression, metabolic activity, proliferation, and adipogenic, calcification, and chondrogenic differentiation potentials before and after preservation.
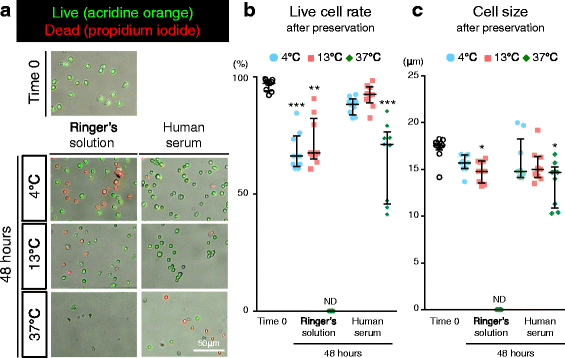
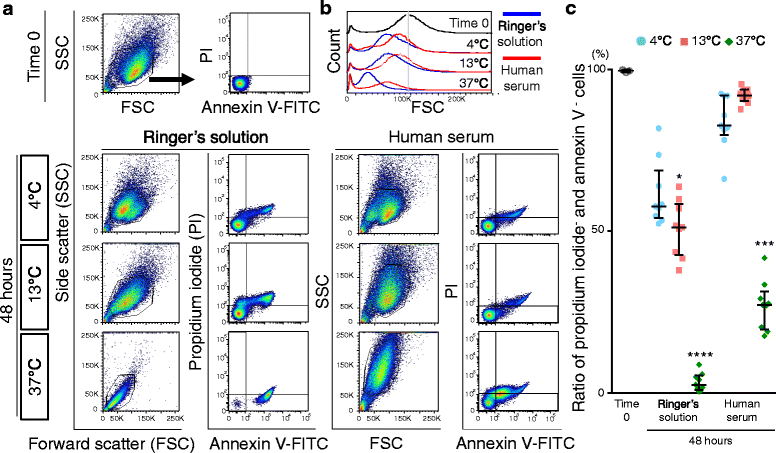
Results: After preservation, synovial MSCs maintained higher live cell rates in human serum than in Ringer's solution at 4 and 13 °C. Synovial MSCs preserved in human serum at 4 and 13 °C also maintained high ratios of propidium iodide- and annexin V- cells. MSC surface marker expression was not altered in cells preserved at 4 and 13 °C. The metabolic activities of cells preserved in human serum at 4 and 13 °C was maintained, while significantly reduced in other conditions. Replated MSCs retained their proliferation ability when preserved in human serum at 4 and 13 °C. Adipogenesis and calcification potential could be observed in cells preserved in each condition, whereas chondrogenic potential was retained only in cells preserved in human serum at 4 and 13 °C.
Conclusion: The viability and chondrogenic potential of synovial MSCs were maintained when the cells were suspended in human serum at 4 and 13 °C.
Keywords: Cell preservation; Human serum; Mesenchymal stem cells; Regenerative medicine; Synovium.
Figures
References
-
- Weisberg HF. Osmotic pressure of the serum proteins. Ann Clin Lab Sci. 1978;8(2):155–64. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

