Atrial-ventricular differences in rabbit cardiac voltage-gated Na+ currents: Basis for atrial-selective block by ranolazine
- PMID: 28610990
- PMCID: PMC5666337
- DOI: 10.1016/j.hrthm.2017.06.012
Atrial-ventricular differences in rabbit cardiac voltage-gated Na+ currents: Basis for atrial-selective block by ranolazine
Abstract
Background: Class 1 antiarrhythmic drugs are highly effective in restoring and maintaining sinus rhythm in atrial fibrillation patients but carry a risk of ventricular tachyarrhythmia. The antianginal agent ranolazine is a prototypic atrial-selective voltage-gated Na+ channel blocker but the mechanisms underlying its atrial-selective action remain unclear.
Objective: The present study examined the mechanisms underlying the atrial-selective action of ranolazine.
Methods: Whole-cell voltage-gated Na+ currents (INa) were recorded at room temperature (∼22°C) from rabbit isolated left atrial and right ventricular myocytes.
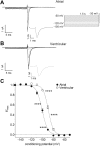
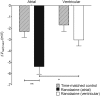
Results: INa conductance density was ∼1.8-fold greater in atrial than in ventricular cells. Atrial INa was activated at command potentials ∼7 mV more negative and inactivated at conditioning potentials ∼11 mV more negative than ventricular INa. The onset of inactivation of INa was faster in atrial cells than in ventricular myocytes. Ranolazine (30 μM) inhibited INa in atrial and ventricular myocytes in a use-dependent manner consistent with preferential activated/inactivated state block. Ranolazine caused a significantly greater negative shift in voltage of half-maximal inactivation in atrial cells than in ventricular cells, the recovery from inactivation of INa was slowed by ranolazine to a greater extent in atrial myocytes than in ventricular cells, and ranolazine produced an instantaneous block that showed marked voltage dependence in atrial cells.
Conclusion: Differences exist between rabbit atrial and ventricular myocytes in the biophysical properties of INa. The more negative voltage dependence of INa activation and inactivation, together with trapping of the drug in the inactivated channel, underlies an atrial-selective action of ranolazine.
Keywords: Antiarrhythmic drug; Atrial myocytes; Cardiac regional heterogeneity; Na(+) channel blocker; Ventricular myocytes.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Carmeliet E., Vereecke J. 1st ed. Kluwer Academic Publishers; New York: 2002. Cardiac cellular electrophysiology.
-
- Lafuente-Lafuente C., Valembois L., Bergmann J.-F., Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;3:CD005049. - PubMed
-
- Camm A.J., Kirchhof P., Lip G.Y.H. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

