Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: Effect of cell seeding density and material stiffness
- PMID: 28611002
- PMCID: PMC5813286
- DOI: 10.1016/j.actbio.2017.06.016
Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: Effect of cell seeding density and material stiffness
Abstract
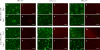
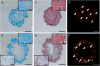
Three-dimensional hydrogel constructs incorporated with live stem cells that support chondrogenic differentiation and maintenance offer a promising regenerative route towards addressing the limited self-repair capabilities of articular cartilage. In particular, hydrogel scaffolds that augment chondrogenesis and recapitulate the native physical properties of cartilage, such as compressive strength, can potentially be applied in point-of-care procedures. We report here the synthesis of two new materials, [poly-l-lactic acid/polyethylene glycol/poly-l-lactic acid] (PLLA-PEG 1000) and [poly-d,l-lactic acid/polyethylene glycol/poly-d,l-lactic acid] (PDLLA-PEG 1000), that are biodegradable, biocompatible (>80% viability post fabrication), and possess high, physiologically relevant mechanical strength (∼1500 to 1800kPa). This study examined the effects of physiologically relevant cell densities (4, 8, 20, and 50×106/mL) and hydrogel stiffnesses (∼150kPa to∼1500kPa Young's moduli) on chondrogenesis of human bone marrow stem cells incorporated in hydrogel constructs fabricated with these materials and a previously characterized PDLLA-PEG 4000. Results showed that 20×106cells/mL, under a static culture condition, was the most efficient cell seeding density for extracellular matrix (ECM) production on the basis of hydroxyproline and glycosaminoglycan content. Interestingly, material stiffness did not significantly affect chondrogenesis, but rather material concentration was correlated to chondrogenesis with increasing levels at lower concentrations based on ECM production, chondrogenic gene expression, and histological analysis. These findings establish optimal cell densities for chondrogenesis within three-dimensional cell-incorporated hydrogels, inform hydrogel material development for cartilage tissue engineering, and demonstrate the efficacy and potential utility of PDLLA-PEG 1000 for point-of-care treatment of cartilage defects.
Statement of significance: Engineering cartilage with physiologically relevant mechanical properties for point-of-care applications represents a major challenge in orthopedics, given the generally low mechanical strengths of traditional hydrogels used in cartilage tissue engineering. In this study, we characterized a new material that possesses high mechanical strength similar to native cartilage, and determined the optimal cell density and scaffold stiffness to achieve the most efficient chondrogenic response from seeded human bone marrow stem cells. Results show robust chondrogenesis and strongly suggest the potential of this material to be applied clinically for point-of-care repair of cartilage defects.
Keywords: Biomaterial scaffold; Bone marrow stem cells; Cartilage tissue engineering; PDLLA-PEG; PLLA-PEG.
Copyright © 2017. Published by Elsevier Ltd.
Figures
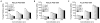
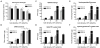
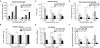
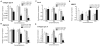

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

