Cases of ALK-Rearranged Lung Cancer with 5-Year Progression-Free Survival with Crizotinib as Initial Precision Therapy
- PMID: 28611010
- PMCID: PMC5659921
- DOI: 10.1016/j.jtho.2017.06.002
Cases of ALK-Rearranged Lung Cancer with 5-Year Progression-Free Survival with Crizotinib as Initial Precision Therapy
Figures
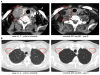
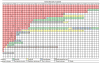
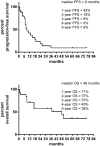
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

