Immunoengineering nerve repair
- PMID: 28611218
- PMCID: PMC5495274
- DOI: 10.1073/pnas.1705757114
Immunoengineering nerve repair
Abstract
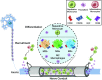
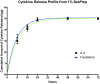
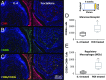
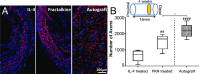
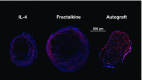
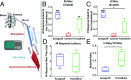
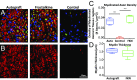
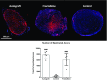
Injuries to the peripheral nervous system are major sources of disability and often result in painful neuropathies or the impairment of muscle movement and/or normal sensations. For gaps smaller than 10 mm in rodents, nearly normal functional recovery can be achieved; for longer gaps, however, there are challenges that have remained insurmountable. The current clinical gold standard used to bridge long, nonhealing nerve gaps, the autologous nerve graft (autograft), has several drawbacks. Despite best efforts, engineering an alternative "nerve bridge" for peripheral nerve repair remains elusive; hence, there is a compelling need to design new approaches that match or exceed the performance of autografts across critically sized nerve gaps. Here an immunomodulatory approach to stimulating nerve repair in a nerve-guidance scaffold was used to explore the regenerative effect of reparative monocyte recruitment. Early modulation of the immune environment at the injury site via fractalkine delivery resulted in a dramatic increase in regeneration as evident from histological and electrophysiological analyses. This study suggests that biasing the infiltrating inflammatory/immune cellular milieu after injury toward a proregenerative population creates a permissive environment for repair. This approach is a shift from the current modes of clinical and laboratory methods for nerve repair, which potentially opens an alternative paradigm to stimulate endogenous peripheral nerve repair.
Keywords: fractalkine; immunomodulation; macrophage; monocyte; nerve repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–160. - PubMed
-
- Millesi H. Factors affecting the outcome of peripheral nerve surgery. Microsurgery. 2006;26:295–302. - PubMed
-
- Bellamkonda RV. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. - PubMed
-
- Giusti G, et al. Return of motor function after segmental nerve loss in a rat model: Comparison of autogenous nerve graft, collagen conduit, and processed allograft (AxoGen) J Bone Joint Surg Am. 2012;94:410–417. - PubMed
-
- Wood MD, et al. Rat-derived processed nerve allografts support more axon regeneration in rat than human-derived processed nerve xenografts. J Biomed Mater Res A. 2014;102:1085–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

