Variable thermal expansion of glass-ceramics containing Ba1-xSrxZn2Si2O7
- PMID: 28611368
- PMCID: PMC5469781
- DOI: 10.1038/s41598-017-03132-x
Variable thermal expansion of glass-ceramics containing Ba1-xSrxZn2Si2O7
Abstract
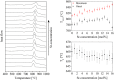
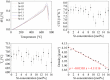
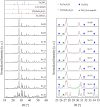
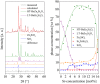
Up to now, the thermal expansion behavior of multiphase glass-ceramics cannot be predicted reliably because of the nescience about the formation of the type and concentration of crystalline phases. In the system BaO-SrO-ZnO-SiO2, recently a new phase based on Ba1-xSrxZn2Si2O7 solid solutions was found, which exhibits unexpected low and highly anisotropic thermal expansion, which can be used for an adjustment of the thermal expansion properties. In the case of sealing materials for high-temperature reactors, the formation of this phase should be avoided. Hence, in this manuscript the concentration thresholds in which these solid solutions precipitate from glasses were determined. The phase analysis was correlated with the thermal expansion behavior of the glass-ceramics. Depending on the Ba/Sr-ratio of the glasses and the considered temperature range, the coefficients of thermal expansion of the glass-ceramics vary between 19.4·10-6 K-1 and 4.8·10-6 K-1. The concentration thresholds in which the as mentioned phases form via crystallization of glasses differ strongly from the literature values obtained via conventional ceramic mixed oxide route.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Reddy AA, Tulyaganov DU, Kharton VV, Ferreira JMF. Development of bilayer glass-ceramic SOFC sealants via optimizing the chemical composition of glasses—a review. J. Solid State Electrochem. 2015;19:2899–2916. doi: 10.1007/s10008-015-2925-5. - DOI
-
- Wang X, Ou DR, Zhao Z, Cheng M. Stability of SrO–La2O3–Al2O3–SiO2 glass sealants in high-temperature air and steam. Ceram. Int. 2016;42:7514–7523. doi: 10.1016/j.ceramint.2016.01.158. - DOI
-
- Luo L, et al. Application of BaO–CaO–Al2O3–B2O3–SiO2 glass–ceramic seals in large size planar IT-SOFC. Ceram. Int. 2015;41:9239–9243. doi: 10.1016/j.ceramint.2015.01.091. - DOI
-
- Fakouri Hasanabadi M, Nemati A, Kokabi AH. Effect of intermediate nickel layer on seal strength and chemical compatibility of glass and ferritic stainless steel in oxidizing environment for solid oxide fuel cells. Int. J. Hydrogen Energy. 2015;40:16434–16442. doi: 10.1016/j.ijhydene.2015.10.023. - DOI
-
- Da Silva MJ, Bartolomé JF, De Aza AH, Mello-Castanho S. Glass ceramic sealants belonging to BAS (BaO–Al2O3–SiO2) ternary system modified with B2O3 addition: A different approach to access the SOFC seal issue. J. Eur. Ceram. Soc. 2016;36:631–644. doi: 10.1016/j.jeurceramsoc.2015.10.005. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

