Identification of an in vivo orally active dual-binding protein-protein interaction inhibitor targeting TNFα through combined in silico/in vitro/in vivo screening
- PMID: 28611375
- PMCID: PMC5469758
- DOI: 10.1038/s41598-017-03427-z
Identification of an in vivo orally active dual-binding protein-protein interaction inhibitor targeting TNFα through combined in silico/in vitro/in vivo screening
Abstract
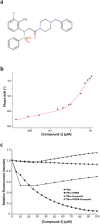
TNFα is a homotrimeric pro-inflammatory cytokine, whose direct targeting by protein biotherapies has been an undeniable success for the treatment of chronic inflammatory diseases. Despite many efforts, no orally active drug targeting TNFα has been identified so far. In the present work, we identified through combined in silico/in vitro/in vivo approaches a TNFα direct inhibitor, compound 1, displaying nanomolar and micromolar range bindings to TNFα. Compound 1 inhibits the binding of TNFα with both its receptors TNFRI and TNFRII. Compound 1 inhibits the TNFα induced apoptosis on L929 cells and the TNFα induced NF-κB activation in HEK cells. In vivo, oral administration of compound 1 displays a significant protection in a murine TNFα-dependent hepatic shock model. This work illustrates the ability of low-cost combined in silico/in vitro/in vivo screening approaches to identify orally available small-molecules targeting challenging protein-protein interactions such as homotrimeric TNFα.
Conflict of interest statement
J.F.Z., R.R., and H.D. are shareholders of Peptinov S.A.S. L.D., H.D., H.M., and G.M. are employed by Peptinov S.A.S.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

