Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis
- PMID: 28611683
- PMCID: PMC5447763
- DOI: 10.3389/fphys.2017.00351
Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis
Abstract
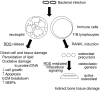
Periodontitis, an inflammatory disease that affects the tissues surrounding the teeth, is a common disease worldwide. It is caused by a dysregulation of the host inflammatory response to bacterial infection, which leads to soft and hard tissue destruction. In particular, it is the excessive inflammation in response to bacterial plaque that leads to the release of reactive oxygen species (ROS) from neutrophils, which, then play a critical role in the destruction of periodontal tissue. Generally, ROS produced from immune cells exhibit an anti-bacterial effect and play a role in host defense and immune regulation. Excessive ROS, however, can exert cytotoxic effects, cause oxidative damage to proteins, and DNA, can interfere with cell growth and cell cycle progression, and induce apoptosis of gingival fibroblasts. Collectively, these effects enable ROS to directly induce periodontal tissue damage. Some ROS also act as intracellular signaling molecules during osteoclastogenesis, and can thus also play an indirect role in bone destruction. Cells have several protective mechanisms to manage such oxidative stress, most of which involve production of cytoprotective enzymes that scavenge ROS. These enzymes are transcriptionally regulated via NRF2, Sirtuin, and FOXO. Some reports indicate an association between periodontitis and these cytoprotective enzymes' regulatory axes, with superoxide dismutase (SOD) the most extensively investigated. In this review article, we discuss the role of oxidative stress in the tissue destruction manifest in periodontitis, and the mechanisms that protect against this oxidative stress.
Keywords: ROS; cytoprotective enzymes; osteoclast; oxidative stress; periodontitis.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

