Peg-Interferon Lambda Treatment Induces Robust Innate and Adaptive Immunity in Chronic Hepatitis B Patients
- PMID: 28611778
- PMCID: PMC5446997
- DOI: 10.3389/fimmu.2017.00621
Peg-Interferon Lambda Treatment Induces Robust Innate and Adaptive Immunity in Chronic Hepatitis B Patients
Abstract
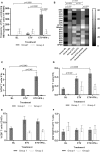
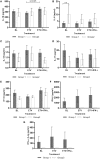
IFN-lambda (IFNλ) is a member of the type III IFN family and is reported to possess anti-pathogen, anti-cancer, and immunomodulatory properties; however, there are limited data regarding its impact on host immune responses in vivo. We performed longitudinal and comprehensive immunosurveillance to assess the ability of pegylated (peg)-IFNλ to augment antiviral host immunity as part of a clinical trial assessing the efficacy of peg-IFNλ in chronic hepatitis B (CHB) patients. These patients were pretreated with directly acting antiviral therapy (entecavir) for 12 weeks with subsequent addition of peg-IFNλ for up to 32 weeks. In a subgroup of patients, the addition of peg-IFNλ provoked high serum levels of antiviral cytokine IL-18. We also observed the enhancement of natural killer cell polyfunctionality and the recovery of a pan-genotypic HBV-specific CD4+ T cells producing IFN-γ with maintenance of HBV-specific CD8+ T cell antiviral and cytotoxic activities. It was only in these patients that we observed strong virological control with reductions in both viral replication and HBV antigen levels. Here, we show for the first time that in vivo peg-IFNλ displays significant immunostimulatory properties with improvements in the main effectors mediating anti-HBV immunity. Interestingly, the maintenance in HBV-specific CD8+ T cells in the presence of peg-IFNλ is in contrast to previous studies showing that peg-IFNα treatment for CHB results in a detrimental effect on the functionality of this important antiviral T cell compartment.
Clinical trial registration: ClinicalTrials.gov NCT01204762.
Keywords: direct antiviral; hepatitis B; immunity; in vivo; peg-interferon lambda.
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

