Proteomic Analysis of Lipid Droplets from Arabidopsis Aging Leaves Brings New Insight into Their Biogenesis and Functions
- PMID: 28611809
- PMCID: PMC5447075
- DOI: 10.3389/fpls.2017.00894
Proteomic Analysis of Lipid Droplets from Arabidopsis Aging Leaves Brings New Insight into Their Biogenesis and Functions
Abstract
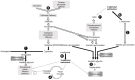
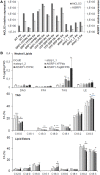
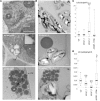
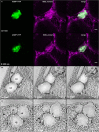
Lipid droplets (LDs) are cell compartments specialized for oil storage. Although their role and biogenesis are relatively well documented in seeds, little is known about their composition, structure and function in senescing leaves where they also accumulate. Here, we used a label free quantitative mass spectrometry approach to define the LD proteome of aging Arabidopsis leaves. We found that its composition is highly different from that of seed/cotyledon and identified 28 proteins including 9 enzymes of the secondary metabolism pathways involved in plant defense response. With the exception of the TRIGALACTOSYLDIACYLGLYCEROL2 protein, we did not identify enzymes implicated in lipid metabolism, suggesting that growth of leaf LDs does not occur by local lipid synthesis but rather through contact sites with the endoplasmic reticulum (ER) or other membranes. The two most abundant proteins of the leaf LDs are the CALEOSIN3 and the SMALL RUBBER PARTICLE1 (AtSRP1); both proteins have structural functions and participate in plant response to stress. CALEOSIN3 and AtSRP1 are part of larger protein families, yet no other members were enriched in the LD proteome suggesting a specific role of both proteins in aging leaves. We thus examined the function of AtSRP1 at this developmental stage and found that AtSRP1 modulates the expression of CALEOSIN3 in aging leaves. Furthermore, AtSRP1 overexpression induces the accumulation of triacylglycerol with an unusual composition compared to wild-type. We demonstrate that, although AtSRP1 expression is naturally increased in wild type senescing leaves, its overexpression in senescent transgenic lines induces an over-accumulation of LDs organized in clusters at restricted sites of the ER. Conversely, atsrp1 knock-down mutants displayed fewer but larger LDs. Together our results reveal that the abundancy of AtSRP1 regulates the neo-formation of LDs during senescence. Using electron tomography, we further provide evidence that LDs in leaves share tenuous physical continuity as well as numerous contact sites with the ER membrane. Thus, our data suggest that leaf LDs are functionally distinct from seed LDs and that their biogenesis is strictly controlled by AtSRP1 at restricted sites of the ER.
Keywords: ER contact site; Small rubber particle protein1; electron tomography; label free proteomics; leaf senescence; lipid droplet; secondary metabolism; ultrastructure.
Figures


References
-
- Attieh J., Kleppinger-Sparace K. F., Nunes C., Sparace S. A., Saini H. S. (2000). Evidence implicating a novel thiol methyltransferase in the detoxification of glucosinolate hydrolysis products in Brassica oleracea L. Plant Cell Environ. 23, 165–174. 10.1046/j.1365-3040.2000.00541.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

