Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction
- PMID: 28611951
- PMCID: PMC5447049
- DOI: 10.3389/fcimb.2017.00216
Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction
Abstract
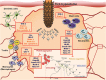
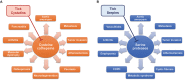
The publication of the first tick sialome (salivary gland transcriptome) heralded a new era of research of tick protease inhibitors, which represent important constituents of the proteins secreted via tick saliva into the host. Three major groups of protease inhibitors are secreted into saliva: Kunitz inhibitors, serpins, and cystatins. Kunitz inhibitors are anti-hemostatic agents and tens of proteins with one or more Kunitz domains are known to block host coagulation and/or platelet aggregation. Serpins and cystatins are also anti-hemostatic effectors, but intriguingly, from the translational perspective, also act as pluripotent modulators of the host immune system. Here we focus especially on this latter aspect of protease inhibition by ticks and describe the current knowledge and data on secreted salivary serpins and cystatins and their role in tick-host-pathogen interaction triad. We also discuss the potential therapeutic use of tick protease inhibitors.
Keywords: cystatins; immunomodulation; protease inhibitors; serpins; tick-host interaction.
Figures
References
-
- Ayllon N., Villar M., Galindo R. C., Kocan K. M., Sima R., Lopez J. A., et al. . (2015). Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis. PLoS Genet. 11:e1005120. 10.1371/journal.pgen.1005120 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources