Deficiency of TYROBP, an adapter protein for TREM2 and CR3 receptors, is neuroprotective in a mouse model of early Alzheimer's pathology
- PMID: 28612290
- PMCID: PMC5645450
- DOI: 10.1007/s00401-017-1737-3
Deficiency of TYROBP, an adapter protein for TREM2 and CR3 receptors, is neuroprotective in a mouse model of early Alzheimer's pathology
Abstract
Conventional genetic approaches and computational strategies have converged on immune-inflammatory pathways as key events in the pathogenesis of late onset sporadic Alzheimer's disease (LOAD). Mutations and/or differential expression of microglial specific receptors such as TREM2, CD33, and CR3 have been associated with strong increased risk for developing Alzheimer's disease (AD). DAP12 (DNAX-activating protein 12)/TYROBP, a molecule localized to microglia, is a direct partner/adapter for TREM2, CD33, and CR3. We and others have previously shown that TYROBP expression is increased in AD patients and in mouse models. Moreover, missense mutations in the coding region of TYROBP have recently been identified in some AD patients. These lines of evidence, along with computational analysis of LOAD brain gene expression, point to DAP12/TYROBP as a potential hub or driver protein in the pathogenesis of AD. Using a comprehensive panel of biochemical, physiological, behavioral, and transcriptomic assays, we evaluated in a mouse model the role of TYROBP in early stage AD. We crossed an Alzheimer's model mutant APP KM670/671NL /PSEN1 Δexon9 (APP/PSEN1) mouse model with Tyrobp -/- mice to generate AD model mice deficient or null for TYROBP (APP/PSEN1; Tyrobp +/- or APP/PSEN1; Tyrobp -/-). While we observed relatively minor effects of TYROBP deficiency on steady-state levels of amyloid-β peptides, there was an effect of Tyrobp deficiency on the morphology of amyloid deposits resembling that reported by others for Trem2 -/- mice. We identified modulatory effects of TYROBP deficiency on the level of phosphorylation of TAU that was accompanied by a reduction in the severity of neuritic dystrophy. TYROBP deficiency also altered the expression of several AD related genes, including Cd33. Electrophysiological abnormalities and learning behavior deficits associated with APP/PSEN1 transgenes were greatly attenuated on a Tyrobp-null background. Some modulatory effects of TYROBP on Alzheimer's-related genes were only apparent on a background of mice with cerebral amyloidosis due to overexpression of mutant APP/PSEN1. These results suggest that reduction of TYROBP gene expression and/or protein levels could represent an immune-inflammatory therapeutic opportunity for modulating early stage LOAD, potentially leading to slowing or arresting the progression to full-blown clinical and pathological LOAD.
Keywords: APP/PSEN1; Alzheimer’s disease; CR3 adapter; Microglia; TREM2 adapter; TYROBP/DAP12.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


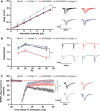
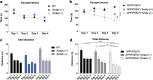
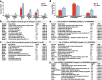
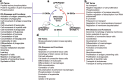

References
-
- Arisi I, Mara D, Brandi R, Felsani A, Capsoni S, Drovandi G, Felici G, Weitschek E, Bertolazzi P, Cattaneo A. Gene expression biomarkers in the brain of a mouse model for Alzheimer’s disease: mining of microarray data by logic classification and feature selection. J Alzheimers Dis. 2011;24:721–738. - PubMed
-
- Benitez BA, Jin SC, Guerreiro R, Graham R, Lord J, Harold D, Sims R, Lambert J-C, Gibbs JR, Bras J, Sassi C, Harari O, Bertelsen S, Lupton MK, Powell J, Bellenguez C, Brown K, Medway C, Haddick PC, van der Brug MP, Bhangale T, Ortmann W, Behrens T, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Haines JL, Turton J, Braae A, Barber I, Fagan AM, Holtzman DM, Morris JC, Williams J, Kauwe JSK, Amouyel P, Morgan K, Singleton A, Hardy J, Goate AM, Cruchaga C. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiol Aging. 2014;35:1510.e19–1510.e26. doi: 10.1016/j.neurobiolaging.2013.12.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

