Outcome of adolescents and young adults compared to children with Hodgkin lymphoma treated with response-based chemotherapy on pediatric protocols: A Children's Oncology Group report
- PMID: 28612375
- PMCID: PMC5799083
- DOI: 10.1002/pbc.26681
Outcome of adolescents and young adults compared to children with Hodgkin lymphoma treated with response-based chemotherapy on pediatric protocols: A Children's Oncology Group report
Abstract
Purpose: We evaluated the outcome of children (<15 years) versus that of adolescents and young adults (AYA; 15-≤ 21 years) treated for Hodgkin lymphoma (HL) in two Pediatric Oncology Group/Children's Oncology Group clinical trials, P9425 and P9426, that used dose-dense, response-based chemotherapy and reduced dose radiotherapy.
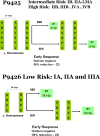
Patients and methods: Subjects 21 years or younger with HL were eligible for these studies. Subjects with low-risk (stages IA, IIA, and IIIA1) without large mediastinal adenopathy biopsy-proven HL, eligible for P9426, were treated with two to four 28-day cycles of doxorubicin, bleomycin, vincristine, and etoposide (ABVE) chemotherapy and 25.5 Gy of involved field radiotherapy. Subjects with intermediate-risk (stages IB, IIA, IIIA1 with large mediastinal adenopathy, and IIIA2) and high-risk (stages IIB, IIIB, and IV) biopsy-proven HL, eligible for P9425, were treated with three to five 21-day cycles of ABVE plus prednisone and cyclophosphamide (ABVE-PC) chemotherapy and 21 Gy of involved region radiotherapy. We compared the 5-year event-free survival (EFS), based on Kaplan-Meier product-limit method, of children versus that of AYA.
Results: Four hundred seventy-one subjects were enrolled on P9425 and P9426 combined. Of these subjects, 203 were AYA, 104 with intermediate and high-risk HL, and 99 with low-risk HL. The 5-year EFS of children did not significantly differ from that of AYA (85.9 vs. 87.1%) with a median follow up of 7.7 years (P = 0.51).
Conclusion: Given the equivalent and excellent results of therapy, HL represents an opportunity for adult and pediatric cancer treatment collaborative groups to jointly design clinical trials targeted to AYA. These trials should focus on both treatment efficacy and the quality of life of AYA while receiving chemotherapy and in reduction of long-term side effects in the survivorship years.
Keywords: AYA; Hodgkin lymphoma; pediatric protocols.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflict of interest pertinent to this publication.
Figures

References
-
- Mauz-Korholz C, Hasenclever D, Dorffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010 Aug 10;28(23):3680–3686. - PubMed
-
- Bonadonna G, Santoro A. ABVD chemotherapy in the treatment of Hodgkin’s disease. Cancer Treat Rev. 1982;9:21–35. - PubMed
-
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003 Jun 12;348(24):2386–2395. - PubMed
-
- Eichenauer DA, Bredenfeld H, Haverkamp H, et al. Hodgkin’s lymphoma in adolescents treatedwith adult protocols: a report from the German Hodgkin study group. J Clin Oncol. 2009 Dec 20;27(36):6079–6085. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

