Effects of branched-chain amino acids (BCAAs) on the progression of advanced liver disease: A Korean nationwide, multicenter, retrospective, observational, cohort study
- PMID: 28614215
- PMCID: PMC5478300
- DOI: 10.1097/MD.0000000000006580
Effects of branched-chain amino acids (BCAAs) on the progression of advanced liver disease: A Korean nationwide, multicenter, retrospective, observational, cohort study
Abstract
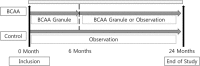
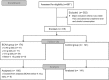
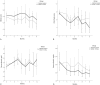
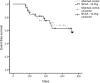
Evidence of the potential benefits of long-term oral branched-chain amino acid (BCAA) supplementation in reducing the severity of liver disease is limited.Patients who were diagnosed with liver cirrhosis with a Child-Pugh (CP) score of 8-10 were included. The BCAA group consumed BCAAs daily for at least 6 months, and the control group consumed a diet without BCAA. We analyzed the improvements based on the model for end-stage liver disease (MELD) score, CP score, incidence of cirrhosis-related complications, and event-free survival over 2 years. Among the 867 recruited patients, 307 (166 in the BCAA group and 141 in the control group) were analyzed. The BCAA group was divided into 3 subgroups, whose patients consumed 4.15 g, 8.3 g, or 12.45 g of BCAAs daily for the analysis. There were significant differences in the CP score, albumin, and hepatic encephalopathy between the 2 groups at baseline. After matching the propensity scores, we analyzed patients in the BCAA-12.45 g group (12.45 g of BCAAs daily, n = 41) and matched control group (n = 41). The MELD score significantly improved in the BCCA-12.45 g group compared to the matched control group (P = .004). The changes in the serum bilirubin level (P = .014) and CP score (P = .033) over time also differed significantly between the 2 groups. The incidence rates of cirrhosis-related complications (P = .973) and development of hepatocellular carcinoma (2 cases each) did not differ significantly between the 2 groups.Long-term oral BCAA supplementation has beneficial effects in patients with advanced liver cirrhosis. A further large-scale prospective study is needed to delineate these beneficial effects.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Effects of Branched-Chain Amino Acid (BCAA) Supplementation on the Progression of Advanced Liver Disease: A Korean Nationwide, Multicenter, Prospective, Observational, Cohort Study.Nutrients. 2020 May 15;12(5):1429. doi: 10.3390/nu12051429. Nutrients. 2020. PMID: 32429077 Free PMC article.
-
Oral branched-chain amino acid granules reduce the incidence of hepatocellular carcinoma and improve event-free survival in patients with liver cirrhosis.Dig Dis. 2011;29(3):326-32. doi: 10.1159/000327571. Epub 2011 Aug 9. Dig Dis. 2011. PMID: 21829025
-
Branched-chain amino acids prevent hepatocarcinogenesis and prolong survival of patients with cirrhosis.Clin Gastroenterol Hepatol. 2014 Jun;12(6):1012-8.e1. doi: 10.1016/j.cgh.2013.08.050. Epub 2013 Sep 10. Clin Gastroenterol Hepatol. 2014. PMID: 24036055
-
Branched-chain amino acid enriched supplements as therapy for liver disease.J Nutr. 2006 Jan;136(1 Suppl):295S-8S. doi: 10.1093/jn/136.1.295S. J Nutr. 2006. PMID: 16365102 Review.
-
Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis.Nutr Clin Pract. 2013 Oct;28(5):580-8. doi: 10.1177/0884533613496432. Epub 2013 Aug 14. Nutr Clin Pract. 2013. PMID: 23945292 Review.
Cited by
-
Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives.J Cancer Prev. 2019 Jun;24(2):72-78. doi: 10.15430/JCP.2019.24.2.72. Epub 2019 Jun 30. J Cancer Prev. 2019. PMID: 31360687 Free PMC article. Review.
-
Molecular Mechanisms and Treatment of Sarcopenia in Liver Disease: A Review of Current Knowledge.Int J Mol Sci. 2021 Jan 31;22(3):1425. doi: 10.3390/ijms22031425. Int J Mol Sci. 2021. PMID: 33572604 Free PMC article. Review.
-
Prospective randomized, placebo-controlled study: role of branched-chain amino acids infusion as adjunct therapy post-liver surgery for patients in the intensive care unit.BMC Gastroenterol. 2025 Jun 19;25(1):439. doi: 10.1186/s12876-025-03696-3. BMC Gastroenterol. 2025. PMID: 40537743 Free PMC article. Clinical Trial.
-
Presence of Sarcopenia and Its Rate of Change Are Independently Associated with Long-term Mortality in Patients with Liver Cirrhosis.J Korean Med Sci. 2018 Oct 31;33(50):e299. doi: 10.3346/jkms.2018.33.e299. eCollection 2018 Dec 10. J Korean Med Sci. 2018. PMID: 30534029 Free PMC article.
-
Therapeutic mechanisms and beneficial effects of non-antidiabetic drugs in chronic liver diseases.Clin Mol Hepatol. 2022 Jul;28(3):425-472. doi: 10.3350/cmh.2022.0186. Epub 2022 Jul 1. Clin Mol Hepatol. 2022. PMID: 35850495 Free PMC article. Review.
References
-
- Swart GR, van den Berg JW, Wattimena JL, et al. Elevated protein requirements in cirrhosis of the liver investigated by whole body protein turnover studies. Clin Sci (Lond) 1988;75:101–7. - PubMed
-
- Mullen KD, Denne SC, McCullough AJ, et al. Leucine metabolism in stable cirrhosis. Hepatology 1986;6:622–30. - PubMed
-
- Marchesini G, Bianchi G, Merli M, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 2003;124:1792–801. - PubMed
-
- Laviano A, Cangiano C, Preziosa I, et al. Plasma tryptophan levels and anorexia in liver cirrhosis. Int J Eat Disord 1997;21:181–6. - PubMed
-
- Henkel AS, Buchman AL. Nutritional support in patients with chronic liver disease. Nat Clin Pract Gastroenterol Hepatol 2006;3:202–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

